Introduction
The NHSScotland National Infection Prevention and Control Manual (NIPCM) was first published on 13 January 2012, by the Chief Nursing Officer (CNO (2012)1), and updated on 17 May 2012 (CNO (2012)1 Update).
The NIPCM provides IPC guidance to all those involved in care provision and is considered best practice across all health and care settings in Scotland.
The re-launch of the NIPCM by the CNO on 11 July 2022 emphasises the ongoing importance of application of Infection Prevention and Control (IPC) guidance within health and care settings across Scotland.
Video of Chief Nursing Officer re-launching the NIPCM
Find out more about the NIPCM
You can find out more about the NIPCM by watching the animation or going to the About the manual webpage.
Disclaimer
When an organisation, for example health and care setting, uses products or adopts practices that differ from those stated in this National Infection Prevention and Control Manual, that individual organisation is responsible for ensuring safe systems of work including the completion of a risk assessment approved through local governance procedures.
Responsibilities
Responsibilities for the content of this manual
ARHAI Scotland must ensure
- that the content of this manual remains evidence based or where evidence is lacking, content is based on consensus of expert opinion.
Stakeholders of the ARHAI Scotland programmes must ensure
- full participation in the working groups and oversight programmes including full engagement with the consultation process outlined in the Terms of Reference associated with each group
Responsibilities for the adoption and implementation of this manual
Organisations must ensure:
- the adoption and implementation of this manual in accordance with their existing local governance processes
- systems and resources are in place to facilitate implementation and compliance monitoring of infection prevention and control as specified in this manual in all care areas
- compliance monitoring includes all staff (permanent, agency and where required external contractors)
- there is an organisational culture which promotes incident reporting and focuses on improving systemic failures that encourage safe infection prevention and control working practices including near misses
Managers of all services must ensure that staff:
- are aware of and have access to this manual
- have had instruction/education on infection prevention and control through attendance at events and/or completion of training (for example via NHS Education for Scotland (NES) and/or local board or organisation)
- have adequate support and resources available to enable them to implement, monitor and take corrective action to ensure compliance with this manual. If this cannot be implemented a robust risk assessment detailing deviations from the manual and appropriate mitigation measures must be undertaken and approved through local governance procedures.
- with health concerns (including pregnancy) or who have had an occupational exposure relating to the prevention and control of infection are timeously referred to the relevant agency, for example General Practitioner, Occupational Health or if required Accident and Emergency
- have undergone the required health checks or clearance (including those undertaking Exposure Prone Procedures (EPPs)
- include infection prevention and control as an objective in their Personal Development Plans (or equivalent)
Staff providing care must ensure that they:
- understand and apply the principles of infection prevention and control set out in this manual
- maintain competence, skills and knowledge in infection prevention and control through attendance at education events and/or completion of training, for example NHS Education for Scotland (NES) and/or local board or organisation
- communicate the infection prevention and control practices to be taken to appropriate colleagues, those being cared for, relatives and visitors without breaching confidentiality
- have up to date occupational immunisations/health checks/clearance requirements as appropriate
- report to line managers and document any deficits in knowledge, resources, equipment and facilities or incidents that may result in transmission of infection including near misses e.g sharps or PPE failures
- do not provide care while at risk of potentially transmitting infectious agents to others - if in any doubt they must consult with their line manager, Occupational Health Department, Infection Prevention and Control Team (IPCT) or Health Protection Team (HPT)
- contact HPT/IPCT if there is a suspected or actual HAI incident/outbreak
Infection Prevention and Control Teams (IPCTs) and Health Protection Teams (HPTs) must:
- engage with staff to develop systems and processes that lead to sustainable and reliable improvements in relation to the application of infection prevention and control practices
- provide expert advice on the application of infection prevention and control in all care settings and provide support to develop individual or organisational risk assessments where deviations from the NIPCM are necessary
- have epidemiological or surveillance systems capable of distinguishing patient case or cases requiring investigations and control
- complete documentation when an incident/outbreak or data exceedence is reported (IPCTs should ensure application of the HIIAT where applicable and report incidents and outbreaks using the ORT as outlined by the HIIAT).
Last updated: 4 October 2021
Chapter 1 - Standard Infection Control Precautions (SICPs)
 Standard Infection Control Precautions (SICPs), covered in this chapter are to be used by all staff, in all care settings, at all times, for all patients1 whether infection is known to be present or not to ensure the safety of those being cared for, staff and visitors in the care environment.
Standard Infection Control Precautions (SICPs), covered in this chapter are to be used by all staff, in all care settings, at all times, for all patients1 whether infection is known to be present or not to ensure the safety of those being cared for, staff and visitors in the care environment.
The Hierarchy of Controls should also be considered in controlling exposures to occupational hazards which include infection risks.
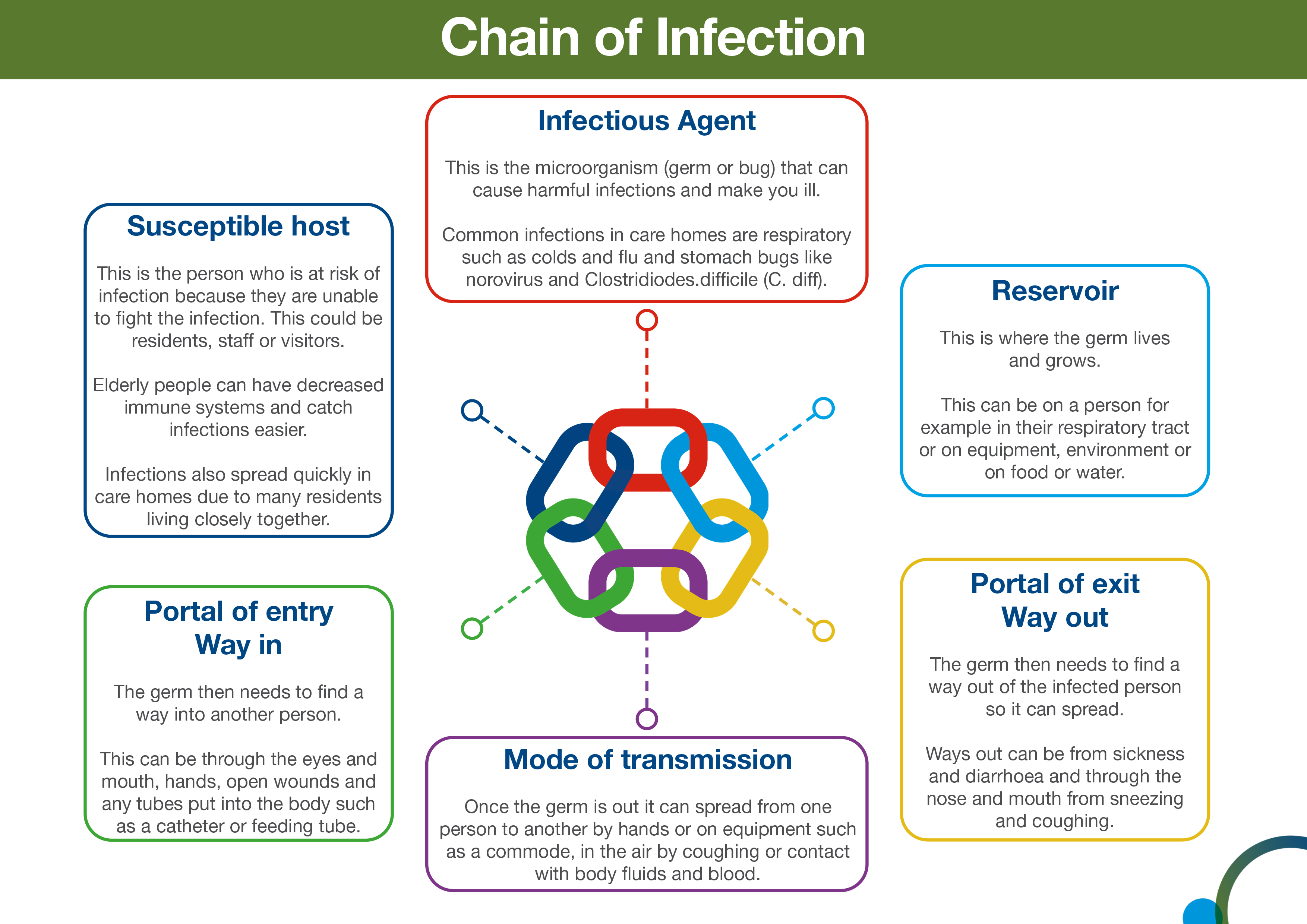
SICPs are the basic infection prevention and control measures necessary to reduce the risk of transmission of infectious agent from both recognised and unrecognised sources of infection.
Sources of (potential) infection include blood and other body fluids secretions or excretions (excluding sweat), non-intact skin or mucous membranes, any equipment or items in the care environment that could have become contaminated and even the environment itself if not cleaned and maintained appropriately.
The application of SICPs during care delivery is determined by an assessment of risk to and from individuals and includes the task, level of interaction and/or the anticipated level of exposure to blood and/or other body fluids.
To be effective in protecting against infection risks, SICPs must be applied continuously by all staff. The application of SICPs during care delivery must take account of:
- risk to and from the individual for whom care is being provided
- the task to be undertaken
- level of interaction
- the anticipated level of exposure to blood and/or other body
Doing so allows staff to safely apply each of the 10 SICPs by ensuring effective infection prevention and control is maintained.
SICPs implementation monitoring must also be ongoing to demonstrate safe practices and commitment to patient, staff and visitor safety.
Further information on using SICPs for Care at Home can be found on the NHS National Education Scotland (NES) website.
1The use of the word 'Persons' can be used instead of 'Patient' when using this document in non-healthcare settings.
Last updated: 28 August 2023
1.1 Patient Placement/Assessment for infection risk
Patients must be promptly assessed for infection risk on arrival at the care area (if possible, prior to accepting a patient from another care area) and should be continuously reviewed throughout their stay. This assessment should influence patient placement decisions in accordance with clinical/care need(s).
Patients who may present a particular cross-infection risk should be isolated on arrival and appropriate clinical samples and screening undertaken as per national protocols to establish the causative pathogen. This includes but is not limited to patients:
- With symptoms such as loose stools or diarrhoea, vomiting, fever or respiratory symptoms.
- With a known (laboratory confirmed) or suspected infectious pathogen for which appropriate duration of precautions as outlined in A-Z pathogens are not yet complete.
- Known or suspected to have been previously positive with a
Multi-drug Resistant Organism (MDRO), for example MRSA, CPE. - Who have been a close contact of a person who has been colonised or infected with CPE in the last 12 months.
- Who have been hospitalised outside Scotland in the last 12 months (including those who received dialysis).
When assessing neonates for infection risk, the mother’s status should be taken into consideration if the mother has:
- been hospitalised outside Scotland in the previous 12 months
- had no antenatal care
- been previously positive with an MDRO, e.g. Meticillin Resistant Staphylococcus Aureus (MRSA) or Carbapenemase Producing Enterobacterales (CPE)
Resources
The Neonatal Assessment for Infection Risk should be used at point of entry or transfer before placement of neonate. The 'Healthcare infections in neonatal units: information for parents and guardians' information leaflet is available.
Further information regarding general respiratory screening questions can be found within the resources section of the NIPCM.
For assessment of infection risk see Section 2: Transmission Based Precautions.
Further information can be found in the patient placement literature review.
1.2 Hand Hygiene
Please note that the term ‘alcohol-based hand rub (ABHR)’ has now been updated to ‘hand rub’. A hand rub (alcohol or non-alcohol based) can be used if it meets the required standards. Please see further information in the hand hygiene products literature review.
Hand hygiene is considered an important practice in reducing the transmission of infectious agents which cause infections.
Adherence with the following points is essential to ensure effective hand hygiene:
- expose forearms (bare below the elbows)
- remove all hand/wrist jewellery* including any embedded jewellery (a single, plain metal finger ring or ring dosimeter (radiation ring) is permitted but should be removed (or manipulated) during hand hygiene). Bracelets or bangles such as the Kara which are worn for religious reasons should be able to be pushed higher up the arm and secured in place to enable effective hand hygiene which includes the wrists
- ensure fingernails are clean, short and that artificial nails or nail products are not worn
- cover all cuts or abrasions with a waterproof dressing
Hand washing should be extended to the forearms if there has been exposure of forearms to blood and/or body fluids.
Hand washing sinks must only be used for hand hygiene and must not be used for the disposal of other liquids. See Chapter 4 - 4.1.4 Management of water outlets including taps and showers).
*Scottish Ambulance Service (SAS) staff should follow this guidance in conjunction with The Association of Ambulance Chief Executives (AACE) position statement: BBE-position-statement-March-2025-V3.0.pdf
To perform hand hygiene
Hand rubs must be available for staff as near to point of care as possible. Where this is not practical, personal hand rub dispensers should be used.
Application of sufficient volume of hand rub to cover all surfaces of the hands is important to ensure effective hand hygiene. Manufacturer’s instruction should be followed for the volume of hand rub required to provide adequate coverage for the hands. In the absence of manufacturers instructions, volumes of approximately 3ml are recommended to ensure full coverage.
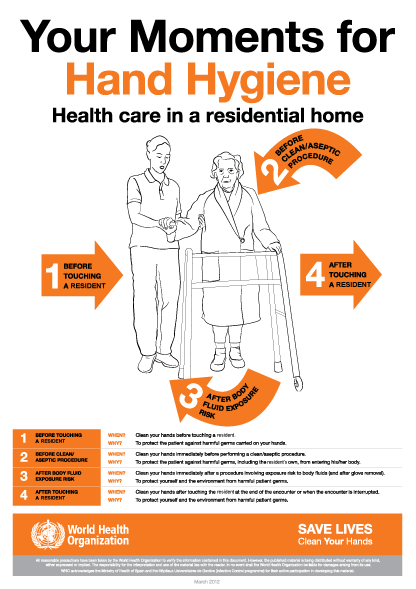
The World Health Organization’s ‘5 moments for hand hygiene’ should be used to highlight the key indications for hand hygiene.
- before touching a patient
- before clean/aseptic procedures. If hand rub cannot be used, then antimicrobial liquid soap should be used
- after body fluid exposure risk
- after touching a patient
- after touching a patient’s immediate surroundings
Some additional examples of hand hygiene moments include but are not limited to:
- before handling medication
- before preparing food
- before donning (putting on) and after doffing (taking off) PPE
- after visiting the toilet
- between carrying out different care activities on the same patient
- after cleaning and disinfection procedures
- after handling waste
Download and print the 5 moments of hand hygiene poster.
Wash hands with non-antimicrobial liquid soap and water if:
- hands are visibly soiled or dirty
- hands are potentially contaminated with blood, other body fluids or excretions
- caring for patients with vomiting or diarrhoeal illnesses
- caring for a patient with a suspected or known gastro-intestinal infection, for example Norovirus or a spore forming organism such as Clostridioides difficile
Hands should be washed with warm/tepid water to mitigate the risk of dermatitis associated with repeated exposures to hot water and to maximise hand washing compliance. Compliance may be compromised where water is too hot or too cold. Hands should be dried thoroughly following hand washing using a soft, absorbent, disposable paper towel from a dispenser which is located close to the sink but beyond the risk of splash contamination.
In all other circumstances use hand rub for routine hand hygiene during care.
Staff working in the community should carry a supply of hand rub to enable them to perform hand hygiene at the appropriate times.
Where staff are required to wash their hands in the service user’s own home they should do so for at least 20 seconds using any hand soap available.
Staff should carry a supply of disposable paper towels for hand drying rather than using hand towels in the individual’s own home. Once hands have been thoroughly dried, hand rub should be used.
The use of antimicrobial hand wipes is only permitted where there is no access to running water. Staff must perform hand hygiene using hand rub immediately after using the hand wipes and perform hand hygiene with soap and water at the first available opportunity.
Resources
(The video above demonstrating Hand Washing and Drying Technique was produced by NHS Ayrshire and Arran)
For how to:
- wash hands see Appendix 1
- hand rub see Appendix 2
Hand hygiene posters and leaflets can be found at Wash Your Hands of Them Resources.
WHO World Hand Hygiene Day 5 May 2025 - It might be gloves. It's always hand hygiene resources are available.
Skin care
- Hand rubs when used for hand hygiene should contain emollients in their formulation.
- Warm/tepid water should be used to reduce the risk of dermatitis. Hot water should be avoided.
- Pat hands dry thoroughly after hand washing using disposable paper towels. Avoid rubbing which may lead to skin irritation/damage.
- Use an emollient hand cream during work and when off duty. These should be applied all over the hands including between the fingers and the back of the hands.
- Do not use refillable dispensers or provide communal tubs of hand cream in the care setting.
- Staff with skin problems should seek advice from occupational health or their GP.
- Barrier creams should not be used in the workplace.
Surgical hand antisepsis
Surgical scrubbing/rubbing applies to persons undertaking surgical and some invasive procedures.
Perform surgical scrubbing/rubbing before donning sterile theatre garments or at other times, for example prior to insertion of central vascular access devices.
Surgical scrubbing using an antimicrobial surgical scrub product should be used for the first surgical hand antisepsis of the day. Or perform hand hygiene using water and a non-antimicrobial liquid soap prior to the first surgical antisepsis of the day, this can be carried out in an adjacent clinical area.
For surgical scrubbing
- Remove all hand/wrist jewellery.
- Nail brushes should not be used for surgical hand antisepsis.
- Nail picks (single-use) can be used if nails are visibly dirty.
- Soft, non-abrasive, sterile (single-use) sponges may be used to apply antimicrobial liquid soap to the skin if licensed for this purpose.
- Use an antimicrobial liquid soap licensed for surgical scrubbing or hand rub licensed for surgical rubbing (as specified on the product label).
- Hand rub can be used between surgical procedures if licensed for this use or between glove changes if hands are not visibly soiled.
- Skin should be blotted dry with sterile single-use towels.
Resources
- For surgical scrubbing technique see Appendix 3.
- For surgical rubbing technique see Appendix 4.
Further information can be found in the Hand Hygiene literature reviews:
- Hand washing, hand rubbing and indications for hand hygiene
- Hand hygiene products
- Skin care
- Surgical hand antisepsis in the clinical setting
1.3 Respiratory and Cough Hygiene
 Respiratory and cough hygiene is designed to minimise the risk of cross-transmission of respiratory illness (pathogens).
Respiratory and cough hygiene is designed to minimise the risk of cross-transmission of respiratory illness (pathogens).
- Cover the nose and mouth with a disposable tissue when sneezing, coughing, wiping and blowing the nose. If a disposable tissue is not available use elbow to cover the nose and mouth when coughing or sneezing.
- Patients showing symptoms of respiratory illness should be encouraged to wear a surgical (TYPE II R FRSM) face mask where it is clinically safe and tolerated by the wearer.
- Dispose of used tissues and face masks promptly into a waste bin.
- In the absence of disposable tissues and hand hygiene facilities only, individuals should cough or sneeze into their elbow/sleeve.
- Wash hands with non-antimicrobial liquid soap and warm water after coughing, sneezing, using tissues, or after contact with respiratory secretions or objects contaminated by these secretions.
- Where there is no running water available or hand hygiene facilities are lacking, staff may use hand wipes followed by hand rub and should wash their hands at the first available opportunity.
- Keep contaminated hands away from the eyes nose and mouth.
Staff should promote respiratory and cough hygiene helping those who need assistance with this, for example elderly and children, providing patients with tissues, plastic bags for used tissues and hand hygiene facilities as necessary.
Resources
Further information can be found in the cough etiquette/respiratory hygiene literature review.
1.4 Personal Protective Equipment
 Before undertaking any care task or procedure staff should assess any likely exposure to blood and/or body fluids and ensure PPE is worn that provides adequate protection against the risks associated with the procedure or task being undertaken.
Before undertaking any care task or procedure staff should assess any likely exposure to blood and/or body fluids and ensure PPE is worn that provides adequate protection against the risks associated with the procedure or task being undertaken.
All PPE should be:
- located close to the point of use
- stored to prevent contamination in a clean/dry area until required for use (expiry dates must be adhered to)
- single-use only items unless specified by the manufacturer, for example reusable items such as non-disposable goggles, face shields or visors
- changed immediately after each patient and/or following completion of a procedure or task
- disposed of after use into the correct healthcare waste stream
Routine sessional use of PPE is not permitted.
Reusable PPE
Reusable PPE items, for example launderable gowns, non-disposable goggles, face shields or visors must be cleaned/decontaminated once removed or placed within a designated container for subsequent cleaning/decontamination with decontamination schedules in place and responsibility assigned.
Reusable PPE must be cleaned/decontaminated as per manufacturers instructions or in line with local policies or procedures.
Resources
Further information on best practice for PPE use for SICPs can be found in Appendix 15.
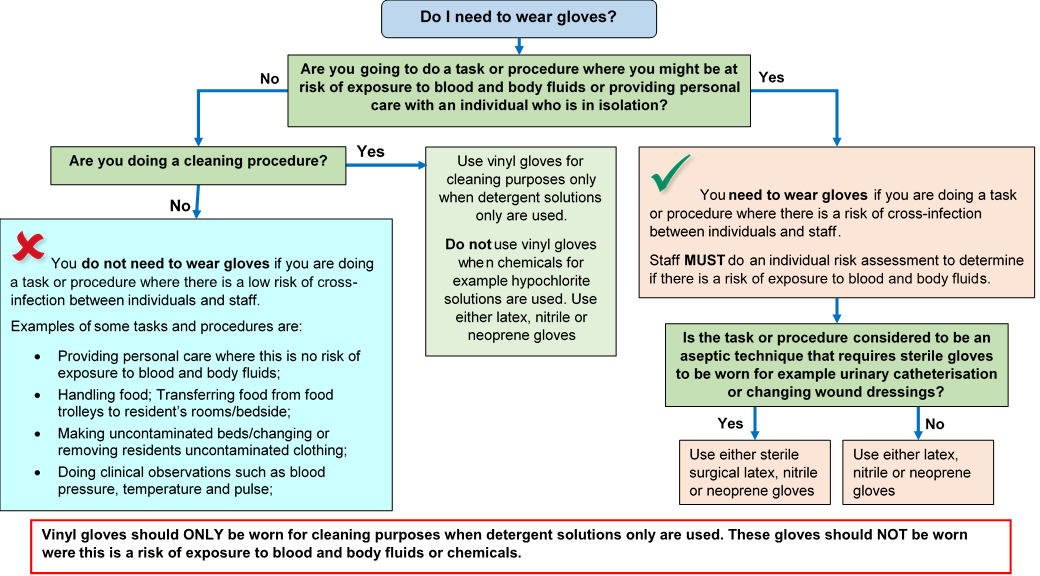
Gloves must:
- be worn when exposure to blood, body fluids, (including but not limited to secretions and/or excretions), non-intact skin, lesions and/or vesicles, mucous membranes, hazardous drugs and chemicals, for example cleaning agents is anticipated/likely. (Scottish National Blood Transfusion Service (SNBTS) adopt practices that differ from those stated in the National Infection Prevention and Control Manual)
- Gloves are a single-use item and should be donned immediately prior to exposure risk and should be changed immediately after each use or upon completion of a task;
- never be worn inappropriately in situations such as to go between patients, move around a care area, work at IT workstations
- be changed if a perforation or puncture is suspected or identified
- be appropriate for use, fit for purpose and well-fitting
- not be worn as a substitute to hand hygiene.
Double gloving is only recommended during some Exposure Prone Procedures (EPPs), for example orthopaedic and gynaecological operations or when attending major trauma incidents and when caring for a patient with a suspected or known High Consequence Infectious disease. Double gloving is not necessary at any other time.
Resources
For appropriate glove use and selection see Appendix 5.
Further information can be found in the Gloves literature review.
Aprons and gowns
The type of apron or gown used in health and care settings should be selected based on the task being undertaken and the anticipated levels of body fluid exposure.
Aprons or gowns should not be worn routinely.
Aprons should be:
- disposable
- fluid resistant
- worn to protect uniform or clothes when contamination with blood, body fluids, chemicals, or cleaning products is likely or when handling used or infectious linen
- worn when in direct care contact with a patient or their immediate environment, for example providing toileting support or changing bed linen
- changed between patients and following completion of a procedure or task
Gowns should be:
- single-use for the task being undertaken
- full-body, long-sleeved, fluid-repellent (either disposable or single-use launderable)
- worn when there is a risk of extensive splashing or extensive contamination with blood or other body fluids
- worn when a disposable apron provides inadequate cover for the procedure/task being undertaken
- changed between patients and immediately after completion of a procedure or task
- considered as part of source control PPE when in close contact with or caring for a patient in protective isolation
Sterile surgical gowns must be:
- worn to prevent contamination of a sterile field during invasive procedures requiring sterile techniques or surgery
- worn by all scrubbed members of the operating theatre surgical team
Launderable gowns:
- should be used for one task or care episode and then be sent for laundering or reprocessing
- should be reprocessed according to the manufacturer’s instructions, including the suggested number of reprocessing cycles and useful life of barrier materials, provided by the manufacturer
- are not be worn in the operating theatre environment or for aseptic surgical procedures
If hand hygiene with soap and water is required, this should not be performed whilst wearing an apron/gown in line with a risk of apron/gown contamination. Hand hygiene using hand rub is acceptable.
A process should be in place for tracking the number of reprocessing cycles and monitoring the quality of reusable gowns to detect any form of deterioration in integrity.
Hand hygiene should be undertaken, if required, using hand rub when wearing an apron or gown. If hands are visibly contaminated and hand hygiene using soap and water must be undertaken while wearing a gown or apron, contamination from water sources must be considered and changing of the apron or gown may be required.
Resources
Further information can be found in the Aprons/Gowns literature review.
Eye/face protection must:
- be worn when there is an anticipated risk of splashing or spraying of blood or bodily fluids and always during Aerosol Generating Procedures
- be compatible with other items of PPE and worn in accordance with manufacturer’s instructions
- not be touched when worn or worn around the neck or on top of the head when not in use
- provide adequate protection from the risks of the task being undertaken
- be removed or changed when vision is impaired due to visible soiling or contamination or damage, when task complete and exposure risk has ended or immediately before leaving the work area
Prescription eyeglasses and contact lenses should not be considered a form of eye or face protection
Resources
Further information can be found in the eye/face protection literature review.
Fluid Resistant Type IIR surgical face masks must be:
- worn by a patient known or suspected to be infected with a micro-organism spread by the droplet or airborne route when leaving their room or when moving between clinical areas including transfers by portering staff and ambulance services
- worn if splashing or spraying of blood, body fluids, secretions or excretions onto the respiratory mucosa (nose and mouth) is anticipated/likely. (As part of SICPs a full-face visor may be used as an alternative to fluid resistant Type IIR surgical face masks to protect against splash or spray)
- worn in combination with a full-face shield, integrated half face shield or goggles for AGPs on non-infectious patients
- worn to protect patients from the operator as a source of infection when performing invasive spinal procedures such as myelography, lumbar puncture and spinal anaesthesia, inserting a Central Vascular Catheter (CVC), performing intra-articular (joint) injections
- worn by all scrubbed members of the theatre surgical team for all surgical procedures
- worn by non-scrubbed members of the theatre surgical team if deemed necessary following a risk assessment of exposure to blood and/or body fluids
- well fitting and fit for purpose (fully covering the mouth and nose)
- removed or changed:
- at the end of a procedure/task
- if the integrity of the mask is breached, e.g. from moisture build-up after prolonged use or from gross contamination with blood or body fluids
- in accordance with specific manufacturers’ instructions
Transparent face masks
Transparent face masks may be used to aide communication with patients in some settings.
Transparent face masks must:
- meet the specification standards of the Transparent face mask technical specification
and
- have been approved by the UK Transparent Mask review group for use within health and social care settings
- only be worn in areas where Fluid Resistant Type IIR surgical face masks are used as personal protective equipment.
Resources
Further information can be found in:
- aerosol generating procedures literature review
- surgical face masks literature review
- section 2.4 of the NIPCM
- appendix 11 of the NIPCM
Footwear should be:
- slip resistant and easy to clean and maintain
- comfortable, soft soled and low heeled with closed toes
- able to protect the foot against spills, potential injury from sharps and contamination
- worn before entering and removed before leaving a care area where dedicated footwear is used, for example theatres. Where dedicated footwear is required, it should not be worn outwith these areas
Employees should clean dedicated footwear daily when in use, if contaminated, and in accordance with local policy or as per manufacturer’s instructions.
Footwear should be replaced when their protective functions are compromised and disposed of in accordance with local waste management protocols
Overshoes or shoe protectors are not generally used within health and care environments. Where their use is required, they should be discarded after each use in accordance with waste management protocols.
Resources
Further information can be found in the footwear literature review.
Headwear must be:
- worn in theatre settings/restricted and semi-restricted areas
- worn as PPE for procedures where splashing/spraying of body fluids is anticipated, and as source control when performing clean/aseptic procedures where risk of infection is deemed to be high
- well-fitting and completely cover the hair
- changed/disposed of at the end of a single clinical procedure/task or at the end of a theatre session (for sessional use): immediately if contaminated with blood and/or body fluids
- removed before leaving the theatre/clean room.
Resources
Further information can be found in the headwear literature review
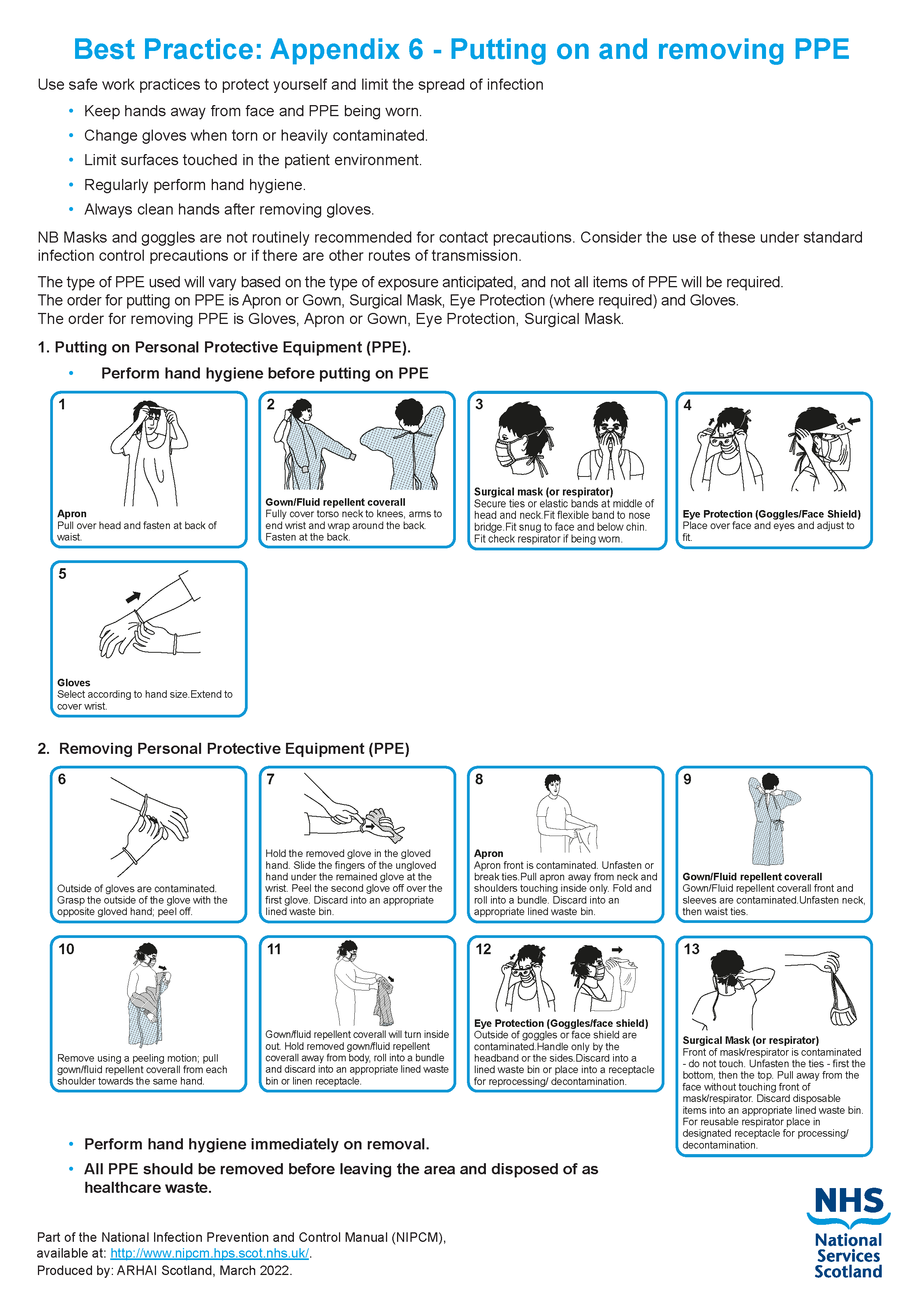
For the recommended method of putting on and removing PPE Appendix 6.
PPE for visitors
Visitors are not routinely required to wear PPE.
If staff or visitors identify the need for PPE, as per the table below, advice on its correct use should be provided by staff.
If a visitor declines to wear PPE when offered, then this should be respected and the visit must not be refused. There is no expectation for staff to monitor the use of PPE by visitors beyond initial advice on its correct use.
The table below shows the PPE which should be worn where appropriate and when the visitor chooses to do so.
IPC Precaution |
Gloves |
Apron |
Face covering/
|
Eye/Face Protection |
|---|---|---|---|---|
|
Standard Infection Control Precautions (SICPs) |
Not required*1 |
Not required*2 |
Where splash/spray to nose/mouth is anticipated during direct care |
Where splash/spray to eyes/face is anticipated during direct care |
*1 unless providing direct care which may expose the visitor to blood and/or body fluids i.e. toileting.
*2 unless providing care resulting in direct contact with the service user, their environment or blood and/or body fluid exposure i.e. toileting, bed bath.
1.5 Safe Management of Care Equipment

Care equipment is easily contaminated with blood, other body fluids, secretions, excretions and infectious agents. Consequently it is easy to transfer infectious agents from communal care equipment during care delivery.
Care equipment is classified as either:
- Single-use – equipment which is used once on a single patient and then discarded. Must never be reused even on the same patient. The packaging carries the symbol below.

- Needles and syringes are single use devices. They should never be used for more than one patient or reused to draw up additional medication.
- Never administer medications from a single-dose vial or intravenous (IV) bag to multiple patients.
- Single patient use – equipment which can be reused on the same patient.
- Reusable invasive equipment - used once then decontaminated for example surgical instruments.
- Reusable non-invasive equipment (often referred to as communal equipment) - reused on more than one patient following decontamination between each use e.g. commode, patient transfer trolley.
Before using any sterile equipment check that:
- the packaging is intact
- there are no obvious signs of packaging contamination
- the expiry date remains valid
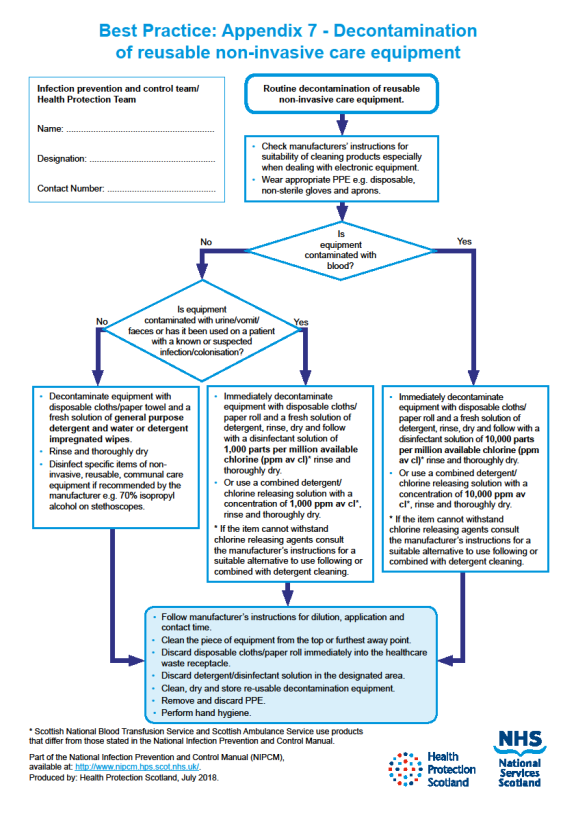
Decontamination of reusable non-invasive care equipment must be undertaken:
- between each use
- after blood and/or body fluid contamination
- at regular predefined intervals as part of an equipment cleaning protocol
- before inspection, servicing or repair
Adhere to manufacturers’ guidance for use and decontamination of all care equipment.
All reusable non-invasive care equipment must be rinsed and dried following decontamination then stored clean and dry.
Decontamination protocols should include responsibility for, frequency of and method of environmental decontamination.
An equipment decontamination status certificate will be required if any item of equipment is being sent to a third-party, for example for inspection, servicing or repair.
Guidance may be required prior to procuring, trialling or lending any reusable non-invasive equipment.
Resources
Further information can be found in the management of care equipment literature review.
For how to decontaminate reusable non-invasive care equipment see Appendix 7.
1.6 Safe Management of Care Environment
 It is the responsibility of the person in charge to ensure that the care environment is safe for practice (this includes environmental cleanliness/maintenance). The person in charge must act if this is deficient.
It is the responsibility of the person in charge to ensure that the care environment is safe for practice (this includes environmental cleanliness/maintenance). The person in charge must act if this is deficient.
The care environment must be:
- visibly clean, free from non-essential items and equipment to facilitate effective cleaning
- well maintained and in a good state of repair
- routinely cleaned in accordance with the Health Facilities Scotland (HFS) National Cleaning Specification:
- a fresh solution of general-purpose neutral detergent in warm water is recommended for routine cleaning. This should be changed when dirty or at 15 minutes intervals or when changing tasks
- routine disinfection of the environment is not recommended. However, 1,000ppm available chlorine should be used routinely on sanitary fittings
- refillable bottles should not be used in settings where immunocompromised patients receive care (haematology and oncology, cardiac surgery, bone marrow and stem cell transplant, neonatal, paediatric and adult ICU, transplant units).
- where refillable bottles are appropriate for use, cleaning products should be freshly made (never topped up) and discarded after 24 hours (or sooner as dependent on manufacturers instructions). The refillable bottle should be washed and thoroughly dried between uses.
Staff groups should be aware of their environmental cleaning schedules and clear on their specific responsibilities.
Cleaning protocols should include responsibility for, frequency of and method of environmental decontamination.
When an organisation adopts decontamination processes not recommended in the NIPCM the care organisation is responsible for governance of and completion of local risk assessment(s) to ensure safe systems of work.
Resources
Further information can be found in the safe management of the care environment (environmental decontamination) literature review.
1.7 Safe Management of Linen
 Clean linen
Clean linen
This is linen that has been processed (laundered) and is ready for use.
- Should be stored in a clean, designated area, preferably an enclosed cupboard.
- Clean linen should be stored separately from used and infectious linen.
- If clean linen is not stored in a cupboard then the trolley or pod used for storage must be designated for this purpose and completely covered with an impervious covering that is able to withstand decontamination.
- Hand hygiene should be performed before handling clean linen.
For all used or infectious linen
Used linen has been used by a non-infectious patient with no visible soiling or contamination by blood or body fluids.
Infectious linen has been used by a patient who is known or suspected to be infectious and/or linen that is contaminated with blood and/or other body fluids for example faeces.
- Any linen used during patient transfer, for example blankets, should be categorised at the point of destination.
- All linen that is deemed unfit for re-use, for example torn or heavily contaminated, should be categorised at the point of use and returned to the laundry for disposal.
When handling used or infectious linen:
- Refer to Appendix 15 for the selection of PPE required.
- Ensure a laundry receptacle is available as close as possible to the point of use for immediate linen deposit.
- Ensure linen is placed in the appropriate receptacle depending on categorisation.
- Check that linen is free from inappropriate items before placing into the laundry receptacle, for example used equipment/needles, service user personal belongings
- Place infectious linen directly into a water-soluble or alginate bag and secure, then place into a plastic bag, for example clear bag, and secure before placing in a laundry receptacle. This applies also to any items heavily soiled and unlikely to be fit for reuse.
- Used and infectious linen bags or receptacles must be tagged, for example: ward or care area and date.
- Perform hand hygiene as recommended in Section 1.2 after handling, bedmaking and bagging linen
Do not:
- rinse, shake or sort linen on removal from beds or trolleys
- place used linen on the floor or any other surfaces, for example a locker or table top
- re-handle used linen once bagged
- overfill laundry receptacles
Store all used or infectious linen in a designated, safe, lockable area whilst awaiting uplift. Uplift schedules must be acceptable to the care area and there should be no build-up of linen receptacles.
Service users, patients and their carers or relatives who are required to take clothing home to launder should be provided with the Washing Clothes at Home Leaflet
Local guidance regarding management of linen may be available.
Resources
Further information can be found in the safe management of linen literature review and National Guidance for Safe Management of Linen in NHSScotland Health and Care Environments - For laundry services/distribution.
Further information about linen bagging and tagging can be found in Appendix 8.
Scottish Government uniform, dress code and laundering policy is available.
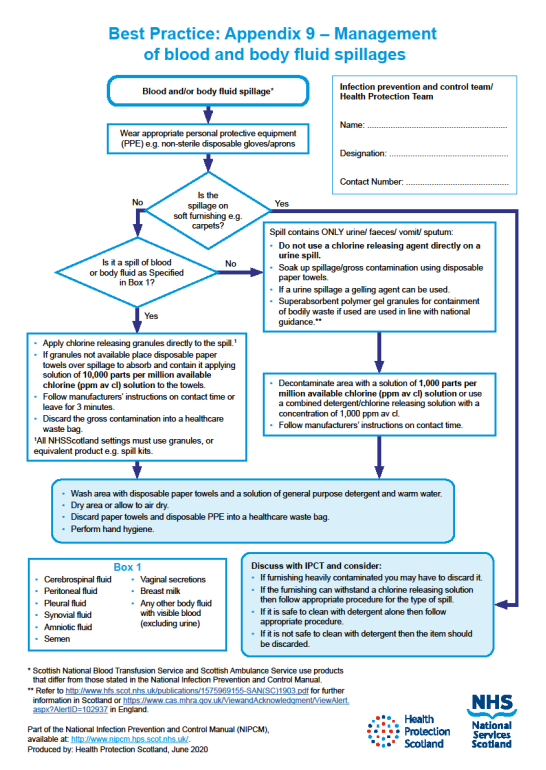
1.8 Safe Management of Blood and Body Fluid Spillages
 Spillages of blood and other body fluids may transmit blood borne viruses.
Spillages of blood and other body fluids may transmit blood borne viruses.
Spillages must be decontaminated immediately by staff trained to undertake this safely.
Responsibilities for the decontamination of blood and body fluid spillages should be clear within each area/care setting.
If superabsorbent polymer gel granules for containment of bodily waste are used these should be used in line with national guidance. In Scotland refer to Safety Action Notice - SAN(SC)19/03 | National Services Scotland (nhs.scot)
Resources
For management of blood and body fluid spillages see Appendix 9.
Further information can be found in the management of blood and body fluid in health and care settings literature review.
1.9 Safe Disposal of Waste (including sharps)
 Scottish Health Technical Note (SHTN)03-01: NHSScotland Waste Management Guidance contains the regulatory waste management guidance for NHSScotland health and care services including waste classification, categorisation, segregation, storage, packaging, transport, treatment and disposal.
Scottish Health Technical Note (SHTN)03-01: NHSScotland Waste Management Guidance contains the regulatory waste management guidance for NHSScotland health and care services including waste classification, categorisation, segregation, storage, packaging, transport, treatment and disposal.
The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation to the safe disposal of sharps.
Waste regulations require the classification of waste based on hazardous characteristics.
Classification of waste
- Special (hazardous) waste. Special waste includes a range of controlled wastes, defined by legislation, which contain dangerous or hazardous substances. Examples of special (hazardous) waste resulting from healthcare activities includes sharps, infectious or potentially infectious clinical waste and some pharmaceuticals or medicinal wastes.
- Non-hazardous waste is residual waste produced in both clinical and non-clinical settings which may include dry recyclates (glass, paper and plastics, metals, cardboard), food waste, packaging waste and furniture.
Healthcare waste should be segregated at source into suitable colour-coded and appropriately labelled receptacles across all health and care settings in Scotland.
SHTN 03-01 contains a full colour-coded waste segregation guide which represents NHSScotland accepted best practice and ensures compliance with current regulations. The most frequently used waste streams in health and care settings are summarised below.
Waste streams
- Black (non-hazardous)
- Residual waste remaining after all source segregated recyclates have been removed.
- For treatment (which may include recovery of materials), then disposal.
- Orange (infectious), light blue (laboratory)
- Orange - consists of infectious or potentially infectious substances or items. Orange lidded leak resistant receptacles may be used for solidified infectious liquids and dialysis waste. Orange bags may be used for items such as PPE, spillage kits, swabs or dressings. Orange lidded sharps box used for sharps disposal only. Consigned to clinical waste treatment facility for treatment and disposal
- Light blue – laboratory/microbiological waste that must be autoclaved before disposal via the orange stream.
- Yellow, red (infectious)
- Yellow or red lidded leak resistant receptacles may be used for waste which poses ethical, highly infectious or contamination risks.
- This includes anatomical and human tissue which is recognisable as body parts but may include other types of waste that require incineration to comply with national or regional policy. Receptacles should be clearly labelled with contents.
- Yellow stream infectious waste is special (hazardous) waste.
- Consigned to clinical waste incineration facility for disposal (high temperature incineration).
Safe waste disposal at care area level
Always dispose of waste:
- immediately and as close to the point of use as possible
- into the correct segregated colour coded approved waste bag or container compliant with UN and relevant industry standards.
Liquid waste, (such as body fluids) that is not suitable for disposal via the toilet or macerator, must be rendered safe by adding a self-setting gel or compound before placing in a rigid leak-resistant receptacle.
Waste bags should not be overfilled and should be securely sealed when 3/4 full (manufacturer’s fill line for sharps boxes) using a closure technique such as a ‘swan neck’ to close with with a plastic tie or tape. The point of origin and date of closure must be clearly marked on the tape/tag or bag.
Store all waste in a designated, safe, lockable area whilst awaiting uplift. Uplift schedules must be acceptable to the care area and there should be no build-up of waste receptacles.
Sharps boxes should:
- have a dedicated handle
- have a temporary closure mechanism, which must be employed when the box is not in use
- be labelled with date of assembly, point of origin and date of closure.
- be disposed of when the manufacturers’ fill line is reached
Local guidance regarding management of waste at care level may be available.
Resources
Further information can be found in the safe disposal of waste literature review.
1.10 Occupational Safety: Prevention and Exposure Management (including sharps)
 Exposure in relation to blood borne viruses (BBV) is the focus within this section and reflects the existing evidence base.
Exposure in relation to blood borne viruses (BBV) is the focus within this section and reflects the existing evidence base.
The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation to:
- arrangements for the safe use and disposal of sharps
- provision of information and training to employees
- investigations and actions required in response to work related sharps injuries
Sharps handling must be assessed, kept to a minimum and eliminated if possible with the use of approved safety devices.
Manufacturers’ instructions for safe use and disposal must be followed.
Needles must not be re-sheathed/recapped.*
Always dispose of needles and syringes as 1 unit.
If a safety device is being used safety mechanisms must be deployed before disposal.
Occupational exposure
An occupational exposure is a percutaneous or mucocutaneous exposure to blood or other body fluids.
Occupational exposure risk can be reduced via application of other SICPs and TBPs outlined within the NIPCM.
Significant occupational exposure
A significant occupational exposure is a percutaneous or mucocutaneous exposure to blood or other body fluids from a source that is known, or found to be positive for a blood borne virus (BBV).
Examples of significant occupational exposures would be:
- a percutaneous injury, for example injuries from needles, instruments, bone fragments, or bites which break the skin
- exposure of broken skin, for example abrasions, cuts, eczema
- exposure of mucous membranes including the eye from splashing of blood or other high risk body fluids
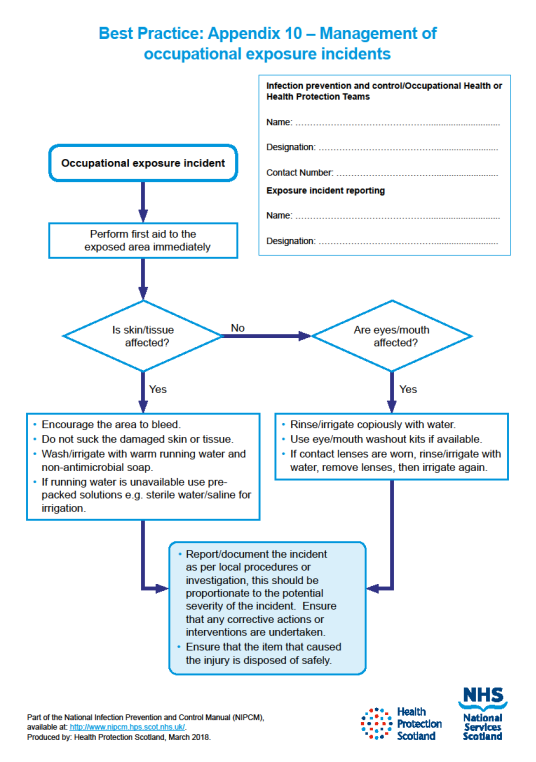
There is a potential risk of transmission of a Blood Borne Virus (BBV) from a significant occupational exposure and staff must understand the actions they should take when a significant occupational exposure incident takes place. There is a legal requirement to report all sharps injuries and near misses to line managers/employers.
Additionally, employers are obligated to minimise or eliminate workplace risks where it is reasonably practicable. Immunisation against BBV should be available to all qualifying staff, and testing (and post exposure prophylaxis when applicable) offered after significant occupational exposure incidents.
Resources
For the management of occupational exposure incidents see Appendix 10
Exposure prone procedures (EPPs)
Exposure prone procedures (EPPs) are invasive procedures where there is a risk that injury to the healthcare worker may result in the exposure of the patient’s open tissues to the blood of the worker (bleed-back).
There are some exclusions for HCWs with known BBV infection when undertaking EPPs. The details of these and further information can be found in the occupational exposure management (including sharps) literature review.
* A local risk assessment is required if re-sheathing is undertaken using a safe technique for example anaesthetic administration in dentistry.
Chapter 2 - Transmission Based Precautions (TBPs)
Transmission-based precautions (TBPs) definitions literature review update
What is changing in this update?
New research questions
New research questions have been considered as part of this update. These look at:
- the historical evidence behind the current transmission descriptors (contact, droplet, airborne) and whether these are still fit for purpose
- how infectious agents are released into the air of the health and care environment from the respiratory tract with consideration of particle size, distance and clearance/fallout time
- the evidence for aerosol generating procedures
New search terms
New search terms have been added. The evidence search goes back to include all scientific papers that have been published since the year 2000.
See the NIPCM literature review development process for more information.
See the full list of research questions considered and the search terms within the revised literature review. Part A of the Considered Judgement Form has been published alongside the revised literature review to provide a summary of the evidence. The review does not contain any recommendations for practice as these are still being developed by the ARHAI Scotland National Policies Guidance and Evidence (NPGE) Working Group.
Due to the scope and complexity of this work, additional external specialist engagement required, and stakeholder involvement to date, ARHAI Scotland have revised the timelines for this review. It is anticipated that the recommendations for practice will be developed during the summer months of 2025, with a revised implementation phase commencing in spring 2026
Evidence for mask wearing
Stakeholders requested that this review also considers where and when healthcare workers should wear masks and the type of mask they should wear (surgical mask or respirator). Evidence for mask effectiveness is being considered in separate literature review updates .
Why are transmission descriptors (contact, droplet, airborne) being reviewed?
The pandemic highlighted that the way in which respiratory transmission is currently described (droplet and airborne transmission) may not reflect what is happening in real life. We need to look at whether there is a better way to describe transmission, and whether this would lead to any improvements in infection prevention and control (IPC) practice.
Understanding how infectious agents are released into the air and the risks associated with particle size and distance from source will help inform this. Reviewing the evidence to understand if there is increased risk associated with certain medical procedures will also inform IPC practice.
The World Health Organization (WHO) and Centers for Disease Control (CDC) have also reviewed transmission descriptors indicating a global shift in the way transmission routes are described. ARHAI Scotland were invited to meet with the WHO global IPC unit to discuss the topic and our literature review findings were well received.
Why were transmission descriptors not reviewed during the pandemic?
Work began on reviewing transmission descriptors in 2022 in the third year of the pandemic. The literature review has assessed over 26,000 scientific articles. 61% of all studies included to answer the main research question were published between 2019 and 2022 which reflects the growing interest in this area as the pandemic continued.
What is likely to change in the NIPCM?
The ARHAI Scotland National Policies Guidance and Evidence (NPGE) Working Group are currently developing recommendations for practice. It is likely that ‘droplet transmission’ and ‘airborne transmission’ will be replaced with new definitions to describe respiratory transmission. This will mean changes throughout the NIPCM to update the terminology including the addition of resources to support any guidance changes.
What might this mean for healthcare workers in practice?
It is too early to understand what might change in practice but it is likely that there will be a need for healthcare workers to consider more factors when risk assessing what PPE to wear.
The goal of the NIPCM is to provide healthcare workers in Scotland with guidance that is evidence based, up-to-date, effective, practical, and as a result, safe. There should be a clear benefit associated with any guidance change and this benefit should outweigh any potential harms. Guidance will only change if these conditions are met.
Supporting resources and education needs will be considered alongside any potential changes to the NIPCM to enable application to practice.
About transmission based precautions (TBPs)
SICPs may be insufficient to prevent cross-transmission of specific infectious agents. Therefore, additional precautions known as transmission based precautions (TBPs) are required to be used by staff when caring for patients with a known or suspected infection or colonisation.
Transmission routes
TBPs are categorised by the route of transmission of infectious agents. Some infectious agents can be transmitted by more than one route.
Contact precautions
Used to prevent and control infections that spread via direct contact with the patient or indirectly from the patient’s immediate care environment (including care equipment). This is the most common route of cross-infection transmission.
Droplet precautions
Used to prevent and control infections spread over short distances (at least 3 feet or 1 metre) via droplets (greater than 5μm) from the respiratory tract of one individual directly onto a mucosal surface or conjunctivae of another individual. Droplets penetrate the respiratory system to above the alveolar level.
Airborne precautions
Used to prevent and control infections spread without necessarily having close patient contact via aerosols (less than or equal to 5μm) from the respiratory tract of one individual directly onto a mucosal surface or conjunctivae of another individual. Aerosols penetrate the respiratory system to the alveolar level.
Application of TBPs
Clinical judgement and decisions should be made by staff on the necessary precautions. This must be based on the:
- suspected or known infectious agent
- transmission route of the infectious agent
- care setting and procedures undertaken
- severity of the illness caused
Appendix 11 provides details of the type of precautions, optimal patient placement, isolation requirements and any respiratory precautions required.
Application of TBPs may differ depending on the setting and the known or suspected infectious agent.
Further information on Transmission Based Precautions can be found in the definitions of Transmission Based Precautions literature reviews.
Last updated: 28 August 2023
2.1 Patient Placement/Assessment for Infection Risk
The potential for transmission of infection must be assessed at the patient’s entry to the care area. If hospitalised or in a care home setting this should be continuously reviewed throughout the stay/period of care. The assessment should influence placement decisions in accordance with clinical/care need(s).
Patients who may present a cross-infection risk in any setting includes but is not limited to those:
- with symptoms such as loose stools or diarrhoea, vomiting, fever or respiratory symptoms.
- with a known (laboratory confirmed) or suspected infectious pathogen for which appropriate duration of precautions as outlined in A-Z of pathogens are not yet complete
- known or suspected to have been previously positive with a Multi-drug Resistant Organism (MDRO), for example MRSA, CPE
- who have been hospitalised (inpatient) outside Scotland in the last 12 months (including those who received dialysis)
Further information regarding general respiratory screening questions can be found within the resources section of the NIPCM.
Isolation facilities should be prioritised depending on the known/suspected infectious agent (refer to Aide Memoire - Appendix 11). All patient placement decisions and assessment of infection risk (including isolation requirements) must be clearly documented in the patient notes.
When single-bed rooms are limited, patients who have conditions that facilitate the transmission of infection to other patients (e.g., draining wounds, stool incontinence, uncontained secretions) and those who are at increased risk of acquisition and adverse outcomes resulting from HAI (e.g., immunosuppression, open wounds, invasive devices, anticipated prolonged length of stay, total dependence on HCWs for activities of daily living) should be prioritised for placement in a single-bed room. Single-bed room prioritisation should be reviewed daily and the clinical judgement and expertise of the staff involved in a patient's management and the Infection Prevention and Control Team (IPCT) or Health Protection Team (HPT) should be sought particularly for the application of TBPs e.g. isolation prioritisation when single rooms are in short supply.
Hospital settings
- Patients who present a cross-infection risk should be isolated in a single room or for patients with a known or suspected pathogen spread by the airborne route, in a specialised negative pressure isolation facility where available.
- Isolation of infectious patients can be in specialised isolation facilities, single room isolation, cohorting of infectious patients where appropriate, ensuring that they are separated by at least 2 metres with the door closed.
- Signage should be used on doors/areas to communicate isolation requirements and prevent entry of unnecessary visitors and non-essential staff.
- Infectious patients should only be transferred to other departments if medically necessary. If the patient has an infectious agent transmitted by the airborne/droplet route, then if possible/tolerated the patient should wear a surgical face mask during transfer.
- Receiving department/hospital and transporting staff must be aware of the necessary precautions.
Cohorting in hospital settings
Cohorting of patients
Cohorting of patients should only be considered when single rooms are in short supply and should be undertaken in conjunction with the local IPCT.
Patients who should not be placed in multi bed cohorts:
- patients with different infectious pathogens/strains and patients with unknown infectious pathogens (laboratory confirmation still awaited)
- patients considered more vulnerable to infection
- patients with a known or suspected infectious pathogen spread by the droplet/airborne route who will undergo an AGP
- patients who are unlikely to comply with TBPs
Staff cohorting
Consider assigning a dedicated team of care staff to care for patients in isolation/cohort rooms/areas as an additional infection control measure during outbreaks/incidents. This can only be implemented through planning of staff rotas if there are sufficient levels of staff available to ensure consistency in staff allocation (so as not to have a negative impact on non-affected patients’ care).
Before discontinuing isolation in hospital settings
Individual patient risk factors should be considered, for example there may be prolonged shedding of certain microorganisms in immunocompromised patients). Clinical and molecular tests to show the absence of microorganisms may be considered in the decision to discontinue isolation and can reduce isolation times. The clinical judgement and expertise of the staff involved in a patient’s management and the Infection Prevention and Control Team (IPCT) or Health Protection Team (HPT) should be sought on decisions regarding isolation discontinuation.
Primary care/out-patient settings
- Patients attending these settings with suspected/known infection/colonisation should be prioritised for assessment/treatment, for example scheduled appointments at the start or end of the clinic session. Infectious patients should be separated from other patients whilst awaiting assessment and during care management wherever possible.
- If transfer from a primary care facility to hospital is required, the ambulance service should be informed of the infectious status of the patient.
Resources
Further information can be found in the patient placement literature review.
2.2 Safe Management of Patient Care Equipment in an Isolation Room/Cohort Area
- Use single-use items if possible.
- Reusable non-invasive care equipment should be dedicated to the isolation room/cohort area and decontaminated prior to use on another patient Section 1.5. Safe Management of Care Equipment
- An increased frequency of decontamination should be considered for reusable non-invasive care equipment when used in isolation/cohort areas.
If an item cannot withstand chlorine releasing agents staff are advised to consult the manufacturer’s instructions for a suitable alternative to use following or combined with detergent cleaning.
Resources
For how to decontaminate non-invasive reusable equipment see Appendix 7.
Note: Scottish Ambulance Service (SAS) and Scottish National Blood Transfusion Service adopt practices that differ from those stated in the National Infection Prevention and Control Manual.
2.3 Safe Management of the Care Environment
Routine environmental decontamination
Hospital setting
Patient isolation/cohort rooms/area must be decontaminated at least daily, this may be increased on the advice of IPCTs/HPTs. These areas must be decontaminated using either:
- a combined detergent/disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl.)) or
- a general purpose neutral detergent in a solution of warm water followed by disinfection solution of 1,000ppm av.cl.
Manufacturers’ guidance and recommended product "contact time" must be followed for all cleaning/disinfection solutions .
Increased frequency of decontamination/cleaning schedules should be incorporated into the environmental decontamination schedules for areas where there may be higher environmental contamination rates, for example
- toilets/commodes particularly if patients have diarrhoea
- “frequently touched” surfaces such as door/toilet handles and locker tops, over bed tables and bed rails
Patient rooms must be terminally cleaned following resolution of symptoms, discharge or transfer. This includes removal and laundering of all curtains and bed screens.
Vacated rooms should also be decontaminated following an AGP.
Primary care/out-patient settings
The extent of decontamination between patients will depend on the duration of the consultation/assessment, the patients presenting symptoms and any visible environmental contamination.
Equipment used for environmental decontamination must be either single-use or dedicated to the affected area then decontaminated or disposed of following use for example cloths, mop heads.
Terminal decontamination
Following patient transfer, discharge, or once the patient is no longer considered infectious.
Remove from the vacated isolation room/cohort area, all:
- healthcare waste and any other disposable items (bagged before removal from the room)
- bedding/bed screens/curtains and manage as infectious linen (bagged before removal from the room)
- reusable non-invasive care equipment (decontaminated in the room prior to removal) Appendix 7.
The room should be decontaminated using either:
- a combined detergent disinfectant solution at a dilution (1,000ppm av.cl.) or
- a general purpose neutral detergent clean in a solution of warm water followed by disinfection solution of 1,000ppm av.cl..
The room must be cleaned from the highest to lowest point and from the least to most contaminated point.
Manufacturers’ guidance and recommended product "contact time" must be followed for all cleaning/disinfection solutions .
Unless instructed otherwise by the IPCT there is no requirement for a terminal clean of an outpatient area or theatre recovery.
Note: Scottish Ambulance Service (SAS) and Scottish National Blood Transfusion Service adopt practices that differ from those stated in the National Infection Prevention and Control Manual.
When an organisation adopts practices that differ from those recommended/stated in the NIPCM with regards to cleaning agents, the individual organisation is fully responsible for ensuring safe systems of work, including the completion of local risk assessment(s) approved and documented through local governance procedures.
2.4 Personal Protective Equipment (PPE): Respiratory Protective Equipment (RPE)
2.4.1 Surgical masks
A type IIR fluid resistant surgical mask should be worn when caring for a patient with a suspected/confirmed infectious agent spread by the droplet route.
Surgical masks worn by patients with suspected/confirmed infectious agents spread by the droplet or airborne routes, as a form of source control, should meet type II or IIR standards.
During periods of increasing or high prevalence of transmissible respiratory infection within healthcare areas, for example emergency departments or throughout a hospital facility, local health boards may undertake a risk assessment and advise wider use of fluid resistant type IIR surgical face masks by healthcare workers in these areas as part of a suite of enhanced control measures. Local epidemiology should be used to inform the risk assessment.
2.4.2 Eye/face protection
Eye and face protection should be worn in combination with:
- a fluid resistant type IIR surgical mask when caring for symptomatic patients infected with droplet transmitted infectious agents
- a fluid resistant FFP3 respirator when caring for symptomatic patients infected with an airborne transmitted infectious agent
Eye and face protection should be worn:
- when there is an anticipated risk of splashing and/or spraying of blood or bodily fluids, and
- when caring for patients with novel infectious agents including pandemic influenza
2.4.3 Aprons/Gowns
The type of aprons or gowns used in health and care settings should be selected based on the task being undertaken, and the anticipated levels of body fluid exposure.
A disposable apron should be worn when in contact with a patient’s environment or when providing direct care to those with known or suspected infection or known or suspected to be colonised/infected with a transmissible infectious agent.
A fluid repellent gown should be used if excessive splashing or spraying is anticipated.
A full body fluid repellent gown should be worn when conducting AGPs on patients known or suspected to be infected with a respiratory infectious agent.
Resources
Further information can be found in the Aprons/Gowns literature review.
2.4.4 Gloves
Gloves must:
- be worn when exposure to blood, body fluids, (including but not limited to secretions and/or excretions), non-intact skin, lesions and/or vesicles, mucous membranes, hazardous drugs and chemicals, e.g. cleaning agents is anticipated/likely
- Gloves are a single-use item and should be donned immediately prior to exposure risk and should be changed immediately after each use or upon completion of a task
- never be worn inappropriately in situations such as to go between patients, move around a care area, work at IT workstations
- be changed if a perforation or puncture is suspected or identified
- be appropriate for use, fit for purpose and well-fitting
- not be worn as a substitute to hand hygiene
Double gloving is only recommended during some Exposure Prone Procedures (EPPs), for example orthopaedic and gynaecological operations, or when attending major trauma incidents and when caring for a patient with a suspected or known High Consequence Infectious disease. Double gloving is not necessary at any other time.
Resources
For appropriate glove use and selection see Appendix 5.
Further information can be found in the Gloves literature review.
2.4.5 RPE
PPE must still be used in accordance with SICPs when using Respiratory Protective Equipment. See Chapter 1.4 for PPE use for SICPs.
Where it is not reasonably practicable to prevent exposure to a substance hazardous to health (as may be the case where healthcare workers are caring for patients with suspected or known airborne micro-organisms) the hazard must be adequately controlled by applying protection measures appropriate to the activity and consistent with the assessment of risk. If the hazard is unknown the clinical judgement and expertise of IPC/HP staff is crucial and the precautionary principle should apply.
Respiratory Protective Equipment (RPE), for instance FFP3 and facial protection, must be considered when:
- a patient is admitted with a known/suspected infectious agent/disease spread wholly by the airborne route
- carrying out aerosol generating procedures (AGPs) on patients with a known/suspected infectious agent spread wholly or partly by the airborne or droplet route
See Appendix 16 for the extant list of Aerosol Generating Procedures which require the application of airborne precautions and details of associated Post AGP Fallow times.
Filter Face Piece 3 (FFP3) Respirators
Where staff have concerns, they may choose to wear an FFP3 respirator rather than a fluid-resistant surgical mask (FRSM) when providing patient care, provided they are fit tested. This is a personal PPE risk assessment.
All tight fitting RPE (for instance FFP3) respirators must be:
- fit tested (by a competent fit test operator) on all healthcare staff who may be required to wear a respirator to ensure an adequate seal/fit according to the manufacturers’ guidance
- fit checked (according to the manufacturers’ guidance) every time a respirator is donned to ensure an adequate seal has been achieved. The poster below gives further information on compatibility of facial hair and FFP3 respirators and can be used when fit testing and fit checking
- single use (disposable) and fluid-resistant. Valved respirators may be shrouded or unshrouded. Respirators with unshrouded valves are not considered to be fluid-resistant and therefore should be worn with a full face shield if blood or body fluid splashing is anticipated
- non valved if a sterile procedure is being performed at the same time as an AGP requiring a respirator to be worn. An MHRA safety alert can be viewed.
- compatible with other facial protection used, for instance protective eyewear, so that this does not interfere with the seal of the respiratory protection. Prescription eyeglasses and contact lenses should not be considered a form of eye/face protection. If wearing a valved, non-shrouded FFP3 respirator a full face shield/visor must be worn
- always put on before entry into the patient room/area and prior to performing an aerosol generating procedure (AGP) and removed in an anteroom/lobby or in a safe area, for example outside the isolation/cohort room/area. All other PPE should be removed in the patient care area
- changed after each use. Other indications that a change in respirator is required include: if breathing becomes difficult, if the respirator becomes wet or moist, damaged or obviously contaminated with body fluids such as respiratory secretions.
Resources
Poster on compatibility of facial hair and FFP3 respirators can be used when fit testing and fit checking.
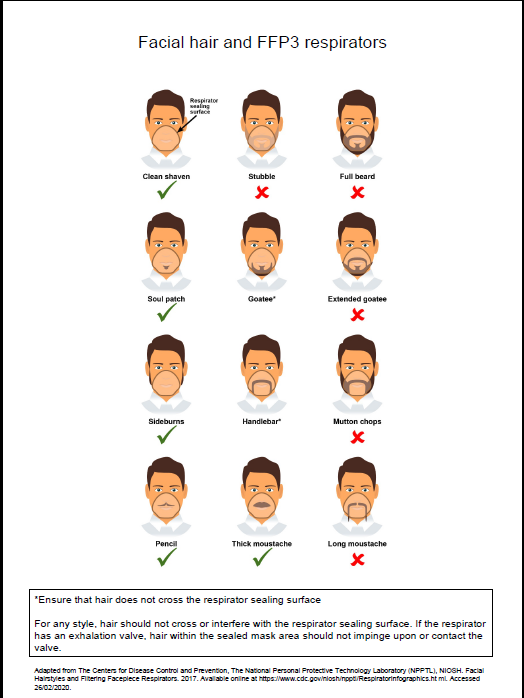
Further information regarding fitting and fit checking of respirators can be found on the Health and Safety Executive website.
National Priority Risk Categorisation for face fit testing with FFP3
The following risk categorisation is the minimum requirement for staff groups that require FFP3 fit testing. NHS boards can add to this for example where high-risk units are present. This categorisation is inclusive of out of hours services.
Level 1 – Preparedness for business as usual
Staff in clinical areas most likely to provide care to patients who present at healthcare facilities with an infectious pathogen spread by the airborne route; and/or undertake aerosol generating procedures. These are A&E, ICU, paediatrics, respiratory, infectious diseases, anaesthesia, theatres, Chest physiotherapists, Special Operations Response Team (Ambulance), A&E Ambulance Staff, Bronchoscopy Staff, Resuscitation teams, mortuary staff.
Level 2 – Preparedness in the event of emerging threat
Staff in clinical setting likely to provide care to patients admitted to hospital in the event of an emerging threat, for example Medical receiving, Surgical, Midwifery and Speciality wards, all other ambulance transport staff.
In the event of an ‘Epidemic/Pandemic’ Local Board Assessment as per their preparedness plans will apply.
The decision to wear an FFP3 respirator/hood should be based on clinical risk assessment, for example task being undertaken, the presenting symptoms, the infectious state of the patient, risk of acquisition and the availability of treatment.
Resources
For a list of organisms spread wholly or partly by the airborne (aerosol) or droplet routes see Appendix 11.
Further information can be found in the aerosol generating procedures literature review.
Powered respirator hoods
Powered respirator hoods are an alternative to FFP3 respirators for example when fit testing cannot be achieved.
Powered hoods must be:
- single use (disposable) and fluid resistant
- the filter must be enclosed with the exterior and the belt able to withstand disinfection with 10,000ppm av.cl.
FFP3 respirator or powered respirator hood
- may be considered for use by visitors if there has been no previous exposure to the infected person or infectious agent; but
- must never be worn by an infectious patient(s) due to the nature of the respirator filtration of incoming air not expelled air.
Work is currently underway by the UK Re-useable Decontamination Group examining the suitability of respirators for decontamination. This literature review will be updated to incorporate recommendations from this group when available. In the interim, ARHAI Scotland are unable to provide assurances on the efficacy of respirator decontamination methods and the use of re-useable respirators is not recommended.
Further information can be found in the Respiratory Protective Equipment (RPE) literature review and the Personal Protective Equipment (PPE) for High Consequence Infectious Diseases (HCIDs) Literature review.
PPE for visitors
PPE may be offered to visitors to protect them from a transmissible infection.
If a visitor declines to wear PPE when it is offered then this should be respected. PPE use by visitors cannot be enforced and there is no expectation that staff monitor PPE use amongst visitors. However, if staff or visitors identify the need for PPE, advice on its correct use should be provided by staff.
When visiting a patient with a known or suspected infection, visitors do not routinely require PPE unless they are providing direct care to the individual they are visiting.
The table below shows the PPE which should be worn where appropriate and when the visitor chooses to do so.
IPC Precaution |
Gloves |
Apron1 |
Face covering/
|
Eye/Face Protection |
|---|---|---|---|---|
|
Transmission Based Precautions (TBPs) |
Not required unless providing direct care which may expose the visitor to blood and/or body fluids i.e. toileting. |
Not required unless providing care resulting in direct contact with the service user, their environment or blood and/or body fluid exposure i.e. toileting, bed bath. |
Where splash/spray to nose/mouth is anticipated during direct care |
Where splash/spray to nose/mouth is anticipated during direct care and/or if within 2 metres of service user with suspected or known respiratory infection |
¹A gown may be selected where excessive splashing or spraying may be anticipated.
2.5 Infection Prevention and Control during care of the deceased
The principles of SICPs and TBPs continue to apply whilst deceased individuals remain in the care environment. This is due to the ongoing risk of infectious transmission via contact although the risk is usually lower than for living patients.
It is important that information on the infection status of the deceased is sought and communicated at each stage of handling. Appropriate risk assessment must be carried out before performing activities that may increase the risk of transmission of infectious agents from deceased individuals (see literature review for further information on these activities).
Washing and/or dressing should not be carried out when the deceased is known or suspected to have been infected by any of the following key infectious agents: Hazard Group 4 organisms, anthrax, and rabies. For other HCIDs a local risk assessment should be undertaken to inform any decision making on washing and/or dressing of the deceased.
Viewing of the deceased should be avoided when the deceased is known or suspected to have been infected by Hazard Group 4 organisms, specifically those causing VHFs (including Ebola, Lassa etc.) and anthrax. For other HCIDs a local risk assessment should be undertaken to inform any decision making on viewing of the deceased.
See Appendix 12 Application of infection control precautions in the deceased.
Staff should advise relatives of the appropriate precautions when viewing and/or having physical contact with the deceased including when this should be avoided.
Deceased individuals known or suspected to have a Hazard Group 4 infectious agent should be placed in a sealed double plastic body bag with absorbent material placed between each bag. The surface of the outer bag should then be disinfected with 1000 ppm av.cl before being placed in a robust sealed coffin.
Post-mortem examination should not be performed on a deceased individual known or suspected to have Hazard Group 4 infectious agents. See Appendix 12 Application of infection control precautions in the deceased. Blood sampling can be undertaken in the mortuary by a competent person to confirm or exclude this diagnosis. Refer to Section 2.4 for suitable PPE.
Post-mortem examination of deceased individuals known or suspected to have been infected by transmissible spongiform encephalopathies (TSE) causing agents should be carried out in such a way as to minimise contamination of the working environment. See Literature review for further information.
Chapter 3 - Healthcare Infection Incidents, Outbreaks and Data Exceedance
The purpose of this chapter is to support the early recognition of potential infection incidents and to guide IPCT/HPTs in the incident management process within care settings; (that is, NHSScotland, independent contractors providing NHS services and private providers of care).
This guidance is aligned to the Management of Public Health Incidents: Guidance on the Roles and Responsibilities of NHS led Incident Management Teams
Built environment incidents/outbreaks
ARHAI Scotland continue to develop evidence-based guidance to further inform Chapter 4 of the National Infection Prevention and Control Manual (NIPCM) on the built environment and decontamination.
Further information is available to support investigation and management of healthcare water-associated infection incidents/outbreaks in Chapter 4.
An Aide-Memoire currently provides best practice recommendations to be implemented in the event of a healthcare ventilation-associated infection incident/outbreak. This will ensure clinical staff, estates and facilities staff, and Infection Prevention and Control Teams (IPCT) have an understanding of the preventative measures required and the appropriate actions that should be taken.
3.1 Definitions of Healthcare Infection Incident, Outbreak and Data Exceedance
The terms ‘incident’ and ‘Incident Management Team’ (IMT) are used as generic terms to cover both incidents and outbreaks
A healthcare infection incident may be:
An exceptional infection episode
- a single case of rare infection that has severe outcomes for an individual AND has major implications for others (patients, staff and/or visitors), the organisation or wider public health for example, high consequence infectious disease (HCID) OR other rare infections such as XDR-TB, botulism, polio, rabies, or diphtheria.
See literature review on PPE for High Consequence Infectious Diseases (HCID).
A healthcare infection exposure incident
- Exposure of patients, staff, public to a possible infectious agent as a result of a healthcare system failure or a near miss e.g. ventilation, water or decontamination incidents.
A healthcare associated infection outbreak
- Two or more linked cases with the same infectious agent associated with the same healthcare setting over a specified time period.
or
- A higher-than-expected number of cases of HAI in a given healthcare area over a specified time period.
A healthcare infection data exceedance
- A greater than expected rate of infection compared with the usual background rate for the place and time where the incident has occurred.
A healthcare infection near miss incident
- An incident which had the potential to expose patients to an infectious agent but did not e.g. decontamination failure.
A healthcare infection incident should be suspected if there is:
- a single case of an infection for which there have previously been no cases in the facility (e.g. infection with a multidrug-resistant organism (MDRO) with unusual resistance patterns or a post-procedure infection with an unusual organism)
Neonatal Units
In addition to using these definitions, neonatal unit investigation by IPCT is also required if:
- a single case of Pseudomonas aeruginosa is identified within a neonatal unit
or
- a single case of infection with an alert organism is identified within a neonatal unit
Additionally, the local IPC team should consider the possibility of any onward transmission and potential for an incident/outbreak where there is:
- A single case of colonisation with an alert organism identified within a neonatal unit.
Resources
Further information can be found in the literature review Healthcare infection incidents and outbreaks in Scotland.
Further information for neonatal IPC management of healthcare incidents and outbreaks can be found in the supporting literature review.
3.2 Detection and recognition of a Healthcare Infection incident/outbreak or data exceedance
An early and effective response to an actual or potential healthcare incident, outbreak or data exceedance is crucial. The local Board IPCT and HPT should be aware of and refer to the national minimum list of alert organisms/conditions. See Appendix 13.
Healthcare associated infection (HAI) Surveillance systems should be used to aid incident/outbreak detection using a combination of retrospective detection of cases alongside prospective enhanced surveillance in high-risk settings (ICU/PICU/NICU, oncology/haematology). A risk-based approach should be applied for other vulnerable groups e.g. cystic fibrosis, oncology and those undergoing renal dialysis.
Local surveillance/reporting systems should be used for recognition and detection of potential healthcare infection incidents/outbreaks within NHS boards. Systems should make use of ‘triggers’ to allow prompt detection of any variance from normal limits.
The Infection Prevention & Control Team (IPCT)/Health Protection Team (HPT) should utilise surgical site infection (SSI) surveillance systems to identify specific post-surgical healthcare infection incidents/outbreaks (in line with national SSI surveillance program as a minimum).
3.2.1 Assessment
Following detection/recognition of an incident/outbreak a member of IPCT or HPT will:
- Undertake an initial assessment, utilising the Healthcare Infection Incident Assessment Tool (HIIAT) - Appendix 14, gather epidemiological data and clinical assessment information on the patient's condition as per:
- NHS boards are required to report all HIIAT assessed Green, Amber and Red reports to ARHAI Scotland through the electronic outbreak reporting tool (ORT). See section 3.2.3.
- NHS boards should monitor the ongoing impact of the incident by escalating and de-escalating as appropriate, using the HIIAT assessment tool. The HIIAT assessment should remain Amber or Red whilst there is ongoing risk of exposure, identification of new cases.
3.2.2 Investigation, management and communication
The IPCT/HPT will establish an IMT if required.
- In the NHS hospital setting the ICD will usually chair the IMT and lead the investigation of healthcare incidents. Where there are implications for the wider community e.g., TB or measles, or rare events such as CJD or a Hepatitis B/HIV look back, or where there is an actual or potential conflict of interest with the hospital service, the CPHM may chair the IMT. A draft agenda for the IMT is available.
- The membership of the IMT will vary depending on the nature of the incident.
- A healthcare infection incident investigation will usually consist of the following elements: an epidemiological investigation, a microbiological investigation and a specific investigation to identify how cases were exposed to the infectious agent (environmental investigation)
- As part of the epidemiological investigation, a case definition(s) must be established by the IMT. A case definition should include the following: the people involved (for example, patients, staff), the symptoms/pathogen/infection (for example, with Group A Streptococci), the place (for example, care area(s) involved) and a limit of time (for example, between January and March year/date). The case definition(s) should be regularly reviewed and refined (if required) throughout the incident investigation as more information becomes available. A working hypothesis regarding the transmission route and source of the exposure must be formed based on initial investigation findings.
- A microbiological investigation into the nature and characteristics of the implicated hazard /infective agent must be conducted.
- Typing and whole genome sequencing can support outbreak and incident investigations. These services are available for some organisms and details of the services available should be discussed with your laboratory. Public Health Scotland continue to offer a SARS-CoV-2 whole genome sequencing service to support outbreak investigations and address important clinical and epidemiological questions.
- An environmental investigation must be conducted if the findings of the epidemiological investigation suggest a common exposure to a potential environmental source/environmental reservoir.
- Review of patient cases should consider any potential missed opportunities to isolate a patient, a delay in which may have resulted in onward transmission. Any learning should be widely communicated to all clinical staff in the board.
- An infection prevention and control assessment to review the existing IPC practices must be conducted, so that areas for immediate improvement can be identified.
- The IMT should receive and discuss all information gathered and epidemiological outputs for example an epidemiological (epi) curve, a timeline and a ward map to:
- determine whether additional case finding and control measures may be necessary
- confirm that all incident control measures are being applied effectively and are sufficient
- Control measures must be directed at the source of the exposure and/or at affected persons in order to prevent secondary/further exposure to the agent. Control measures must be initiated within 24 hours of receiving the initial report and should be implemented based on relevant guidance (for example pathogen specific) and investigation findings of the nature of the outbreak.
- A follow-up period may be defined after an infection incident/outbreak has ended to ensure its termination, including assessment of any ongoing control measures and would be determined by the PAG/IMT.
- Identify any change(s) in the system: staffing, procedures/processing, equipment, suppliers. A step-by-step review of procedure(s). An outbreak checklist is available.
- Identify and count all cases and/or persons exposed: This includes the total number of confirmed/probable/possible exposed cases. An incident/outbreak data collection tool is available.
If staff screening is being considered as part of the investigation DL (2020)1 must be followed.
- HAI deaths, which pose an acute and serious public health risk, must be reported to the Procurator Fiscal, refer to SGHD/CMO(2018)11.
- The IMT must ensure affected patients, and where appropriate their next of kin, have been informed of any actual or potential harm as a result of the HAI. Duty of Candour must be considered at each IMT.
- All significant adverse event reviews involving a category 1 adverse event (events that may have contributed to or resulted in permanent harm, for example unexpected death) should also be reported.
- If no new cases arise and any remaining cases are considered to no longer pose a risk, the IMT should agree on actions prior to resumption of normal service.
3.2.3 Communications
- Following the PAG/IMT, the NHS Board is required to communicate all HIIAT Green, Amber and Red assessments with ARHAI Scotland, by completing the electronic Outbreak Reporting Tool (ORT) within 24 hours of HIIAT assessment.
- Exported MS Excel files must be emailed to ARHAI Scotland for processing – the “Export Data File for ARHAI” button within the ORT only saves the extract from the ORT into the folder. Extracted data files should be emailed to the ARHAI Scotland ICT mailbox (NSS.ARHAIinfectioncontrol@nhs.scot).
- The Protocol for the Reporting of Healthcare Infection Incidents, Outbreaks and Data Exceedance in NHSScotland through the Outbreak Reporting Tool (ORT) is available in the resources section of the NIPCM.
- For incidents/outbreaks that are HIIAT assessed as Red, Amber or Green, frequency of updates are as follows:
- HIIAT Red – review, update and submit a daily update.
- HIIAT Amber– review, update and submit a twice weekly update.
- HIIAT Green – review, update and submit a weekly update.
- The ORT form is for any HIIAT Red, Amber or Green assessed incident/outbreaks. Incidents assessed as Red, Amber or Green, where ARHAI support is requested, will be reviewed for onward communication to Scottish Government Healthcare Associated Infection Policy Unit.
- Respiratory incidents/outbreaks associated with key respiratory pathogens (COVID-19, influenza and respiratory syncytial virus (RSV)), should be completed within the Respiratory Short Form. However, where IPC measures do not align with the outbreak checklist and NIPCM, or where ARHAI support is requested a full ORT form must be completed. The Outbreak Checklist is available in the resources section of the NIPCM website.
- COVID-19 reporting should now align with reporting for other key respiratory pathogens (Influenza/RSV).
- Any adverse event related to equipment or medication must be reported as soon as possible (within one working day) to the Incident Reporting and Investigation Centre (IRIC) and the escalation/de-escalation flowchart followed.
Closure of incident/outbreak with lessons learned
- Once the incident is declared over, and in addition to reporting via the electronic outbreak reporting tool (ORT), the IMT/NHS board should decide on the most appropriate format for a report. This is to communicate any lessons learned using the Hot Debrief Tool. Completion of this and submission to ARHAI Scotland is not mandatory, but for the purposes of sharing lessons learned across Scotland.
The IMT Chair, in discussion with the IMT, should determine whether further reporting on the incident and the incident management is required i.e. SBAR Report and full IMT report template are available in the resources section of the NIPCM website.
Chapter 4 - Infection Control in the Built Environment and Decontamination
The healthcare built environment means the buildings and related infrastructure used for the provision of healthcare. This includes utilities such as water and ventilation and any fixed or semi-permanent components within the healthcare facility with which patients, visitors and staff will interact.
The healthcare built environment plays a critical role in the provision of safe patient care and protection to staff and visitors . Chapter 4 is informed by evidence-based literature reviews and best practice guidance tools and resources relating to IPC in the built environment.
4.1 Water
Vulnerable (high-risk) patients may be at a greater risk of infection from water system-associated organisms following exposure to water within healthcare settings during the delivery of healthcare.
It is important that the potential infection risks from water systems are understood by those delivering care. This chapter sets out guidance to help reduce this risk.
4.1.1 High-risk patients
High-risk patients
High-risk patients are those who are vulnerable to infection as a result of a compromised immune system, underlying health conditions and associated medical treatments.
High-risk patient groups should include as a minimum:
- haematology
- oncology
- cardiac surgery
- bone marrow and stem cell transplant patients
- neonatal, paediatric and adult intensive care unit (ICU) patients
- solid organ transplant
- burns
- cystic fibrosis patients
- any other patients that are severely immunocompromised through disease or treatment
The types of infection that water system-associated organisms may cause include:
- bloodstream (including CVC-associated bloodstream infection)
- respiratory (pneumonia)
- skin and soft tissue (including insertion site infections around any invasive device)
- surgical site infection (for example endocarditis after cardiac surgery, wound infections)
- urinary tract infection (UTI)
- disseminated disease
Patients may become infected, colonised before they become infected or may be colonised with no active infection.
4.1.2 Responsibilities
Water Safety Group (WSG)
Each NHS board should have a multidisciplinary water safety group. SHTM 04-01 part B describes in detail the role, responsibilities and membership of the WSG.
As a minimum this WSG should:
- have in place board water safety plans (WSP)
- identify high-risk patient groups and areas across all healthcare settings and include these in the WSP
- high-risk patients can receive care or treatment out with high-risk settings (for example theatres or radiology) and this should also be considered within the WSP
- review all uses of water within healthcare settings and include these in water safety plans
- review risk assessments and water safety plans on a pre-agreed frequency (minimum annually) and when there are alterations, repairs, changes of use, building works, and incidents.
- have ongoing input throughout any new build or refurbishment project process including commissioning, handover and any associated HAI-SCRIBE
- have general oversight of water quality across the NHS board area
- confirm that water is of potable quality and that any other minimum testing requirements are being met
- ensure routine water testing is being undertaken where required
- review results, provide assurance and/or act upon out of specification water testing results
- ensure that clear responsibilities are defined for the interpretation, dissemination and actions of water results
- be assured that flushing requirements across the NHS board are being met including those areas which have low or altered occupancy and any which are unoccupied
- have an agreed plan regarding point of use (POU) filters which includes their requirement, selection, fitting, ongoing maintenance and their review
- ensure that HCWs, patients and care givers are supported to comply with and understand the importance of safe use of water practices
- comply with the roles and responsibilities as described in SHTM 04-01 Part B
4.1.3 Safe clinical care
Safe use of water
It is important that the potential HAI risks from water systems are understood by those delivering care, specifically the potential transmission routes for water system-associated organisms, which can include:
- direct contact with water, for example oral care, washing and bathing, hydrotherapy
- indirect contact with contaminated equipment, medical products, staff and other patients
- aerosolisation from aerosols generated from the process of water splashing and spraying, and aerosols released from contaminated water-based equipment for example cardiopulmonary bypass machines and heater-cooler units used during cardiac surgery
- aspiration from inhalation of contaminated water into the airways, usually by patients that are intubated, via nasogastric tubes (where the contaminated water has been used to prepare the food), those requiring oral fluid replacement and those requiring orally administered medications
Minimise patient contact with tap water
Staff should consider the location and proximity of high-risk patients to tap water, drains and any associated splashing or spraying.
Alternatives to tap water, such as cleansing wipes, hand rub and water free shampoos should be considered while taking into account patient needs, patient choice and infection risk.
Use of sterile water
- For severely immunocompromised patients, for example allogeneic stem cell transplant patients, sterile water should be considered for drinking, oral care and washing.
- Sterile water should be considered to replace tap water for washing babies within neonatal settings specifically for babies that:
- are under 28 weeks gestation
- have non-intact skin
- have invasive devices
- are in humidified incubators
Milk
Powdered infant formulas should be prepared using freshly boiled water according to manufacturer’s instructions.
Frozen breast milk should be defrosted in one of the following ways:
- using a water-free warming device
- in a designated fridge
- at room temperature
Any water free warming devices should be single patient use, and stored in an appropriate, clean and patient identifiable container, with a fitted lid.
Once defrosted, any unused milk should be discarded in accordance with local waste policy and never disposed of via a clinical wash hand basin (CWHB).
Use of ice
Installation of ice machines should be by approval of the Water Safety Group (WSG) and in accordance with manufacturer instructions and SHTM 04-01 Part A . A WSG approved cleaning, maintenance and audit schedule should also be in place.
Ice for consumption
- Ice for consumption by high-risk patients should not be made using ice-making machines.
- Where ice is required for consumption in these patient groups, it should be made by putting drinking water into single-use ice-making bags and frozen in a conventional freezer.
- Alternatively, iced water may be provided by freezing single bottles of commercially available spring water and allowing patients to drink that ice water as it melts.
Ice for treatment
- Where ice is required for treatment purposes by high-risk patients, it should not be made using an ice machine. It should be made using water obtained through a microbiological point of use (POU) filter, sterile water, or cooled boiled water in single-use ice-making bags and frozen in a conventional freezer.
- Conventional freezers used in healthcare should be maintained and cleaned in line with manufacturer’s instructions with an agreed cleaning, maintenance and audit schedule in place.
4.1.4 Management of water outlets including taps and showers
- Clinical sinks including wash hand basins - safe practice points
- Showers
- Surface contamination from spraying or splashing during water outlet use
- Flushing
Water outlets that are used infrequently or not at all may present a transmission risk from stagnant water and have the ability to contaminate the wider water system. Consideration should be given to removal of the outlet where there is no longer a clinical need for it. See SHTM 04-01 Parts A & B for further information.
Clinical sinks including wash hand basins – safe practice points
Below is an educational animation which focuses on clinical wash hand basins (CWHBs), their intended purpose, water associated infection risks, and what we all can do together to reduce this risk. The animation is supported by a poster for use beside CWHBs to locally promote good practice for health and care staff as well as the general public who may visit those settings.
Animation: Good practice for clinical wash hand basins
Poster: Good practice for clinical wash hand basins
- All clinical sinks and CWHBs should conform to SHTM 64.
- There should be enough space in the sink to allow the required activity to be performed without touching the basin sides, fixtures, or fittings (for example, taps), including whilst point of use (POU) filters are installed.
- When filling equipment such as patient wash bowls these items should not touch the taps, POU filter, basin sides or drain outlet.
- Water flowing from the tap should not be able to flow directly into the drain as this can cause splashing.
- Hand hygiene product dispensers should be placed so that the contents cannot leak or spill into/onto water outlets.
- During ongoing water quality issues, use hand rub as standard for hand hygiene. If hands are visibly soiled, contaminated with blood or body fluids or if gastro-intestinal infection is suspected or confirmed in the patient:
- wash with soap and water
- dry thoroughly
- then perform hand hygiene with hand rub
- Clinical wash hand basins should only be used for the purpose of performing hand hygiene.
- Clinical wash hand basins should not be used for disposal of any foodstuffs, drinks, bodily fluids, clinical or medicinal waste.
- Do not wash or place medical or patient care equipment in clinical wash hand basins, patient sinks, showers or baths.
- Equipment should not be stored on or near to the sink.
- Personal items (for example toothpaste, cosmetics) should not be stored on the sink.
Showers
- Shower heads and hoses should be allowed to drain after use so that water is not retained in a loop in the hose
- The shower hose should not be long enough to allow the shower head to touch the shower drain or floor.
- Showers should be included in the flushing programme.
Staff should report any problems or concerns regarding the safety, maintenance, usage, and cleanliness of water outlets to the appropriate service for example estates and facilities department/ancillary staff.
Surface contamination from spraying or splashing during water outlet use
Water flowing from taps during use should not create any splashing onto surrounding surfaces or equipment.
- Drug preparation, aseptic or other clinical procedures should not be carried out in close proximity to sinks, water outlets or within surrounding areas where splashing may occur.
- Splash zones may occur up to 2 metres from the water outlet, however risks should still be considered in areas where splash or spray exceeds this distance to manage these risks locally.
- Other factors to consider include the environmental layout, location of equipment (fixed and moveable), placement of patients, whether patients are high risk, and the activities to be undertaken.
- If there is splashing of surrounding areas and relocation or reconfiguration of the water outlet is not possible, physical barriers such as impermeable, wipeable screens capable of withstanding cleaning agents can be considered.
Flushing
Flushing of taps should be undertaken at all outlets (See SHTM 04-01 Part B) including little used outlets and outlets within low occupancy areas.
All departments should identify a responsible person to ensure that flushing of all outlets are being performed in their areas as specified, in practice this may be the Senior Charge Nurse, Clinical Lead or domestic manager.
- In high-risk settings, all outlets should be flushed at least daily for one minute.
- In all other settings, all outlets should be flushed twice weekly as a minimum for at least three minutes in occupied buildings and should be based on local risk assessment, taking into account the local water pressure, temperature and flow rate.
- For any outlet currently fitted with a POU filter, the filter should not be removed in order to undertake flushing.
- Outlet flushing should not cause splashing/spraying beyond the outlet. If flushing creates splashing or spraying onto adjacent surfaces, the area should be cleaned/disinfected (as per NIPCM Chapter 1) to reduce the risk of contamination and slippages and falls.
- Records should be maintained to demonstrate that flushing has been undertaken and for the appropriate duration.
- Using a pre-existing daily schedule to incorporate flushing, for example the local domestic cleaning schedule, can increase compliance.
- All staff involved with flushing responsibilities should be appropriately trained.
4.1.5 Water dependent equipment
Water dependent equipment includes any items of equipment that comes into direct or indirect contact with a patient or their environment and also uses or requires to process or hold water to function.
Water dependent equipment, is a potential infection source and should be considered as potential sources for infection as part of outbreak or incident investigations.
Neonatal incubators
- Sterile water (not tap water) should be used within humidifiers for neonatal incubators in accordance with manufacturers instructions.
- Neonatal incubators (including mattresses) should be completely dismantled, cleaned, decontaminated and thoroughly dried between patients (or every 7 days when used continuously by the same patient), using cleaning products that are compatible with the equipment and in accordance with manufacturer’s instructions. Any moisture left within or on the incubator can encourage microbial growth.
- The re-usable reservoirs of humidified incubators should be cleaned and sterilised between uses in a central decontamination unit, if manufacturer guidance allows.
Cardiac heater cooler units
- Health and care settings should refer to NHSScotland Guidance for Decontamination and testing of Cardiac Heater Cooler Units (HCUs).
Refillable bottles
- Refillable bottles should not be used in high-risk settings where patients receive care.
- Where appropriate for use, refillable bottles should be washed and thoroughly dried and protected from environmental contamination. Any moisture may promote the growth of microorganisms.
- Products used in refillable bottles should be freshly made (never topped up) and discarded after 24 hours (or sooner as dependent on the solution). Manufacturer’s instructions should also be followed.
4.1.6 Point of Use (POU) filters
A POU filter is an external filter that is fitted to a water outlet (taps and showers) to filter out microorganisms and debris.
A POU filter does not remove or kill the microorganism, but traps micro-organisms allowing for the safe use of the water.
The water safety plan (WSP) approved by the WSG should state the types of POU filter which can be used. The WSG should agree local processes and responsibilities for the requirement, installation, maintenance, management and removal of POU filters.
- Installation of POU filters may be considered by the WSG or an IMT where a potential or actual clinical risk has been identified as being associated with the water.
- Ensure when a POU filter is fitted there is no additional splashing or spraying of water and that water does not flow directly into the drain.
- POU filters should be replaced as per manufacturer instructions or if visibly contaminated, damaged, or leaking.
- POU filters may be removed when the Water Safety Group and/or IMT determines that water quality can be maintained without the use of the filter.
- Once the POU filter is removed the outlet and its associated pipework should be immediately cleaned and flushed to remove any accumulated debris.
4.1.7 Water testing
- Water testing at commissioning
- Routine water testing
- Frequency of testing
- Microbiological limits
- Selection of outlets for sampling
- Interpretation of routine water testing results
It is important that responsibilities for routine and ad hoc water testing are well defined by the Water Safety Group.
Each NHS board should have processes in place for the receipt, reporting and distribution of results which should include as a minimum:
- escalation of out of specification results. Exceptions should be recorded and rapidly disseminated to all WSG members and local IPC team (a record should be kept of distribution lists for reporting)
- clear responsibilities should be defined for interpretation and action of results
Water testing at commissioning
- In order to ensure IPC input and oversight of risk, IPC teams should be involved in the design and planning process and engaged through to commissioning and handover. This includes agreeing acceptable water sampling parameters and remedial actions to be taken if results are unacceptable.
- A full set of the analysed water sample results should be approved by the WSG before handover and before the system is brought into clinical use
- The WSG should confirm water is of potable quality and meets other minimal water testing requirements with clinical and microbiological oversight from the ICD/microbiologist on behalf of the WSG.
- After water system replacement/remedial activities, water sample analysis results should be approved by the IMT/ WSG or agreed local process.
What to test
A sampling plan with appropriate microbiological parameters and should be agreed by WSG prior to tender. As a minimum it should include testing in all settings for:
- Total Viable Counts (TVCs)
- coliform bacteria (including E.coli)
- Legionella spp.
Testing for P. aeruginosa should be carried out in high-risk settings.
Local risk assessment should determine if there are additional testing requirements.
When to test
Samples should be taken no sooner than five days and no later than seven days after a full disinfection process has been completed and a further set of samples should be taken immediately prior to handover.
- Analysis should be carried out by an independent accredited laboratory (United Kingdom Accreditation Service (UKAS) or ISO 9002). See SHTM 04-01 Part C.
Actions following commissioning results
As part of their commissioning water safety plan, the NHS board should have pre-agreed processes in place should the results of commissioning tests be unsatisfactory. For more details on commissioning, see SHTM 04-01 Part A and BS 8680.
Routine water testing
Results from routine water testing over time (trend analysis) provides evidence of effective control measures and also supports early detection of HAI risks.
What to test
The WSG should agree the routine water testing required and this should form part of the water safety plan. As a minimum:
- routine water testing should be undertaken for Pseudomonas aeruginosa and Legionella species in high-risk settings and areas where high-risk patients are treated
- a local risk assessment according to BS 8580-1 and BS 8580-2 should be undertaken to assess the need for routine water testing in other care areas and for organisms other than Legionella and Pseudomonas aeruginosa
- consider trend analysis for Total Viable Counts (TVCs) to monitor water quality as this may indicate when results are deviating from what is considered normal for that particular water system
- equipment and/or medical procedures that use water which is separate from the main hot and cold water distribution system should be routinely tested in line with relevant guidance/manufacturer’s instructions which includes:
- water for heater cooler units (NHSScotland Guidance for Decontamination and testing of Cardiac Heater Cooler Units (HCUs)
- water used for renal dialysis
- Where no UKAS accreditation exists for routine testing of specific healthcare water system-associated microorganisms, boards should still consider testing. The UKAS Technical Bulletin highlights the following important points.
- There will always be new and emerging organisms causing outbreaks, for which there will not yet be established or accredited methods but where microbiology testing laboratories play a vital role in determining the source of infection.
- There is significant risk to patients if sources during outbreaks are not detected and mitigated against. The lack of UKAS accreditation for a specific test does not preclude laboratories from processing these samples. Such specimens can be processed provided the laboratory states on the report that the test is not UKAS accredited.
Frequency of testing
As a minimum, testing for P. aeruginosa and Legionella spp. in high-risk settings should occur every 6 months.
The frequency of routine microbiological water testing in other areas and for other microorganisms should be based on a comprehensive risk assessment undertaken by the WSG.
Increases to the frequency of water testing should occur:
- during a suspected or confirmed outbreak where a link to the water system is being explored or considered
- if surveillance identifies an increased incidence of infection known or suspected to be associated with the water system
- after implementing any changes to the water system for example after biocide dosing, remedial works or a refurbishment project
- when thermal or chemical controls have failed or are failing (for example when levels of biocide are lower than the agreed limit)
Consideration should be given to increasing the frequency of routine water testing when pre-flush trend analysis demonstrates increasing colony forming units (cfu)/100 ml for P. aeruginosa.
Microbiological limits
Healthcare water systems
Recommended microbiological limits for water samples are detailed in the table below.
Note: Incubate drinking water system samples at 22˚C and 37˚C for 24 hours in accordance with BS EN ISO 6222.
Pathogen |
Colony Forming Units (CFU) |
|---|---|
| Coliform bacteria (including Escherichia coli) | 0 cfu/100ml |
| Enterococci | 0 cfu/100ml |
| P. aeruginosa | 0 cfu/100ml |
| Legionella spp. | Undetectable in high-risk units. <100 cfu/litre in non-high-risk units |
| Legionella pneumophila serogroup 1 (Lp1) | Undetectable |
| For all other gram-negative healthcare water system-associated organisms | 0 cfu/ml |
Additional microbiological limits
The table below details recommended additional microbiological limits for water samples obtained from water dependent equipment.
Procedure |
Colony Forming Units (CFU) |
Total Viable Count(TVC) |
Endotoxin |
|---|---|---|---|
| Heater cooler unit water | 0 cfu/100ml for Mycobacterium spp. | TVC cut-off levels of <100 cfu/ml | None |
| Hydrotherapy water | <20 cfu/litre for Legionella spp. 0 cfu/100ml for Staphylococcus aureus as part of wider investigations only (local decision) |
TVC cut-off levels of <10 cfu/ml | None |
| Endoscopy final rinse water | 0 cfu/100ml for Mycobacterium spp. | TVC cut-off levels of <10 cfu/100 ml | Endotoxin limit of <0.25 EU/ml |
| Final rinse water in surgical instrument washer disinfectors | TVC cut-off levels of <1 cfu/100 ml | None | Endotoxin limit of <0.25 EU/ml |
| Renal dialysis fluid and water | TVC cut-off levels of <50 cfu/ml | None | Endotoxin limit of <0.125 EU/ml |
Selection of outlets for sampling
For routine testing, samples collected should be pre-flush only unless otherwise directed by the WSG/IPCT or Microbiology.
- Pre-flush sampling in a busy ward may have to occur early in the morning or at another time when water outlet usage is lower.
- Pre-flush samples should be taken by collecting water straight from the outlet as it is first turned on.
- Pre-flush samples may help to identify colonisation in a particular outlet.
If post-flush samples are to be taken, the sample should be collected after first running the outlet to flush through the pipes. Post-flush samples may support differentiation between local and systemic colonisation following a positive pre-flush result (see BS 7592 for more information).
A planned targeted sampling plan should be developed by the WSG to include:
- up-to-date schematic of the systems, including identified sampling points
- risk assessment and identification of water sampling requirements which ensure areas identified as high-risk in terms of patient susceptibility are included
- high-risk also includes areas supporting microorganism growth for example cooler parts of the hot water system or warmer parts of the cold water system
Take samples from the proximal and distal ends of each water system with a locally agreed number of sampling points between.
- The number of samples collected during any single round of sampling should be sufficient to be fully representative of the entire water distribution system.
- The outlets being sampled within clinical facilities should be rotated at each sampling round unless a decision has been made to sample all outlets every time.
- Outlets within ancillary facilities (including those shared by departments) such as staff kitchens, domestic services rooms (DSR), treatment rooms and preparation rooms, should be tested during every round of routine sampling.
Interpretation of routine water testing results
- Responsibilities for receiving and initial interpretation of water results should be agreed by the WSG and included in the WSP. This should include agreed processes for escalation of out of specification results.
- Any test results which are above the agreed microbiological limits should be escalated for attention by the Infection Prevention and Control Doctor and Consultant Microbiologist and the AE (water) where required.
- The clinical risk associated with the sample location should be considered when interpreting results.
- If water test results are above the microbiological limits, the following environmental factors should be reviewed to assist with interpretation of results (the water system’s schematic diagram may support this process):
- water temperature
- pH
- residual disinfectant
- water softeners
- water turnover
- Routine water test results should be interpreted as a series of trends (over time) and with an awareness of the systems schematic and current condition.
Sampling results and actions following a non-compliant result (no patient cases)
- If coliforms are identified in a water sample, a repeat sample should be collected and tested to rule out a false positive.
- Whenever pre-flush sample results remain above the microbiological limits, pre- and post-flush samples should be collected to determine if there is a local or systemic contamination. Where post-flush samples remain above microbiological limits, it may indicate systemic contamination. Negative or low post-flush samples may indicate a local contamination (outlet and/or associated pipework and/or fittings near the outlet).
- Taking water samples from further back in the system (beyond the outlet itself) should be considered when positive pre-flush and post-flush sample test results are obtained.
- Following a positive water test result, an immediate review of existing control measures and risk assessment by the IPC team and estates team should be carried out to identify additional remedial/clinical actions required.
- If water continues to test positive following remedial intervention at the outlets, consider taking water samples from further back in the system (beyond the outlet itself).
- Repeat samples should be taken when disinfection/remedial actions have taken place to ensure effectiveness of actions.
Remedial actions following positive water results (no patient cases)
Remedial actions should be agreed by the WSG.
Remedial actions should be determined based on consideration of the water test results in context with the water system as a whole, any existing control measures, and the areas where the result has been obtained.
- When post-flush samples are negative or have low counts, remedial actions should be directed towards the outlet (and associated pipework and fittings).
- When attempting to remove or reduce microbial contamination at the outlet (inclusive of the drain) consider:
-
- disinfection (chemical and/or heat treatment),
- physical replacement of parts of the outlet
- removal of the entire outlet (including dead-legs)
- If contamination is suspected to extend beyond the outlet (further back in the system) whole system water disinfection may be required.
For more information on whole water system disinfection see (SHTM 04-01 part D ‘Disinfection of Domestic Water Systems’
4.1.8 Indications for routine environmental surface sampling
Routine environmental surface sampling may be beneficial in addition to routine water sampling if seeking to determine the extent of environmental contamination.
Knowledge of environmental sources of contamination can support development of measures to prevent transmission from those sources to patients.
Environmental sources include any sites that are exposed to water.
- When deciding on the need for, and frequency of, routine environmental surface sampling, past incidents or outbreaks may help identify specific locations within the healthcare facility where contamination was previously detected or where relevant patient colonisations and infections occurred. This information allows for targeted sampling in areas that may pose a higher risk of transmission.
Environmental surface sampling can also be used to measure the effectiveness of any decontamination methods in use.
See 4.1.9 incident investigation for information on environmental surface sampling in response to clinical cases.
Actions following environmental surface sampling results
- Results interpretation should be undertaken by the ICD/microbiologist/IMT.
- Results may indicate the need to implement or strengthen control measures for example cleaning, decontamination, replacement of equipment/fixtures, and change to clinical practice/patient care.
4.1.9 Incident investigation
- Preparedness
- Detection of a healthcare-associated infection incident or outbreak
- Investigation
- Water testing in response to clinical cases
- Environmental surface sampling in response to clinical cases
- Closing an incident
Preparedness
The water safety group should have an agreed WSP that covers all care settings and is inclusive of a business continuity/contingency arrangement in preparation for the event that a water source, for example mains water, system water, tap water, cannot be used or an area cannot be used due to widespread known or suspected contamination.
All high-risk settings should have a setting-specific alert organism list for clinical isolates, which should be informed by the known historical epidemiology of that setting and allow for early identification of single cases of unusual environmental organisms.
As a minimum, this alert organism list should include
- Acinetobacter spp.
- Burkholderia spp.
- Chryseomonas indologenes
- Cupriavidus pauculus
- Legionella spp.
- Pseudomonas spp.
- Mycobacterium abscessus
- Mycobacterium chelonae
- Mycobacterium fortuitum
- Mycobacterium chimaera
- Mycobacterium mucogenicum
- Serratia marcescens
- Sphingomonas spp.
- Stenotrophomonas maltophilia
See Appendix 13 for more information on alert organisms including the locations/patient cohorts to which each apply.
Detection of a healthcare-associated infection incident or outbreak
Colonisation or infection of gram-negative microorganisms or non-tuberculous mycobacteria, isolated from a clinical sample in any patient should raise a high degree of suspicion of a healthcare associated environmental link and should be investigated/reviewed.
An environmental source should be considered when Enterobacteriaceae is isolated from a clinical sample and a data exceedance has been identified.
Isolation of Legionella spp. from a clinical sample in any patient indicates transmission from the environment and should be investigated as a possible healthcare associated infection incident if the incubation period fits and there is no established link to a community source. Further information can be found in the NIPCM A-Z of Pathogens for Legionella spp.
When determining HAI status, the incubation period should be considered, acknowledging the wide variation (a few hours to years) for environmental organisms.
- The incubation period for environmental organisms, particularly in high-risk groups may be relatively short and may not fit with the routinely applied 48 hour rule.
- Careful consideration should be applied when assessing an HAI in this category, recognising also that whilst a patient is receiving antibiotics which may assist in selecting the gram-negative organism more readily, it should still be considered and investigated.
Environmental organism outbreaks and incidents may occur over extended periods of time with significant time between cases. Consideration of an environmental link should be given to cases that have been identified over a wide time period.
Ensure that the risk of a false negative result is considered and further sampling discussed. This can be supported by taking a measured approach to sampling from the outset.
To assist with the management of incidents or outbreaks involving healthcare water system-associated organisms, a checklist is available in the resources section.
Investigation
- A multi-disciplinary IMT chaired by the ICD/ Consultant in Public Health Medicine (CPHM) should be established when any water associated infection risk is identified, to support the board and WSG to manage the incident.
- Healthcare water system incidents should be assessed according to the NIPCM healthcare Infection Incident Assessment Tool (HIIAT). For more information see Chapter 3 of the NIPCM.
- The Incident Management Team (IMT) should consider all possible sources including the environment (the water supply itself plus the plumbing components) and patients as both may be sources that can lead to ongoing transmission of waterborne infectious agents to other patients and further contamination of the environment.
- If it is essential for the water outlets to remain in use, point of use (POU) filters should be installed while investigations are ongoing and remedial actions are being considered.
Water testing in response to clinical cases
The IMT should agree a water sampling plan to identify and prioritise potential sources taking account of the following:
- the local water system for example the wash hand basin/shower in the patient's room
- mains water supply
- the layout and schematics of the associated water system
- water associated equipment and procedures and any other sources of water exposure during patient care
- the potential involvement of refillable bottles
- historical water testing results/ trends
- known epidemiological information (including historical)
- the geographical distribution of any infected or colonised cases (during their entire healthcare journey)
Water samples should be taken before disinfection of the water system or equipment or before any other remedial actions are initiated.
As a minimum, a pre-flush sample should be taken from each outlet being sampled. Post-flush samples should also be considered.
Sampling instructions can be found in SHTM 04-01 Part C
- For sampling guidance specific to Legionella spp., BSI 7592:2022 ‘Sampling for Legionella bacteria in water systems – Code of practice’ should be followed.
Where no UKAS accreditation exists for specific healthcare water system-associated organisms, boards should still consider testing and can seek advice from ARHAI Scotland. See the UKAS Technical Bulletin for more information.
Environmental surface sampling in response to clinical cases
Environmental surface sampling (swabbing) should be carried out when there is more than one working hypothesis and an environmental source is suspected.
- Consider data exceedance (for example sporadic cases of colonisation or infection over a set time period which might occur over many years).
- An environmental sampling plan should be agreed by the IMT.
- Identify and prioritise potential sources or reservoirs taking account of:
- epidemiological information (including historical)
- historical environmental and water sampling results
- the geographical distribution of the infected and colonised cases throughout their entire healthcare journey
- Environmental sites would include any sites that are exposed to water.
- Environmental samples should be taken before environmental cleaning or decontamination occurs or any other environmental remedial actions have been initiated.
Closing an incident
When considering whether to declare an infection incident or outbreak as ‘closed’ or ‘over’ the IMT should be assured that transmission risks have been mitigated, including exposure from any remaining colonised or infected patients and that there is a surveillance plan in place to allow for early detection of any further potentially linked cases.
Healthcare Water Systems Associated Organisms Incident or Outbreak Checklist
This checklist is an adaptation of the 2015 Pseudomonas aeruginosa Outbreak Checklist and should be used pending the development of new resources.
It aligns with the findings of the Infection prevention and control for safe water systems literature review and should be used in conjunction with the guidance contained within Chapter 4 of the National Infection Prevention and Control Manual (NIPCM).
Bed spacing
Guidance consistently recognises that bed spacing requirements contribute towards the control of HAIs. All NHS boards and care providers should aim to meet the minimum bed spacing requirements laid out in the guidance below and in keeping with the date of design and construction of the building. This takes account of ergonomics within the clinical environment and not just healthcare associated infection (HAI) risk. Some other health and care settings may choose to adopt this guidance e.g. hospice settings.
Adult in-patient facilities designed post 2010 should achieve 3.6m (width) x 3.7m (depth) dimensions of SHPN 04-01, HBN 00-03 and SHFN 30. Width of 3.6m is measured from bed centre to bed centre. Since 2014, HBN 00-03’s Figure 45 states a day treatment bay should achieve 2.45m width/centre-to-centre dimension.
Current NHS Scotland Guidance on bed spacing is listed below:
- Core guidance - General design for healthcare buildings (HBN 00-01)
- Core guidance - Clinical and clinical support spaces (HBN 00-03)
- Critical care units (HBN 04-02)
- HAI-SCRIBE Manual information for project teams (SHFN 30 Part A)
- HAI-SCRIBE Implementation strategy and assessment process (SHFN 30 Part B)
- HAI-SCRIBE question sets and checklists (SHFN 30 Part C)
- Adult in-patient facilities (SHPN 04-01)
- In-patient accommodation - supp 1 - Isolation facilities in acute settings (SHPN 4 sup 1)
Clinical Assurance
ARHAI Scotland’s Clinical Assurance programme provides clinical and infection prevention and control (IPC) expertise to complement the work undertaken by the NHSScotland’s Assure (NHSSA) assurance service.
Key Stage Assurance Reviews (KSAR) focus on ensuring infection prevention and control is a key consideration for healthcare construction projects. ARHAI Scotland have developed notes for NHS Board IPC Teams which aim to help navigate this process.
More information about the programme and a list of tools and guidance developed is available.
Publications
Work undertaken and published to date has been cited here for ease of reference and use at a clinical level.
Many of these publications were produced prior to development of chapter 4 and were published outwith the existing manual methodology.
Updates to publications will be made where required as part of the ARHAI programme work plans.
ARHAI Scotland will work with SG directorates responsible for these areas in planning to establish planned implementation.
Decontamination
- NHSScotland Guidance for decontamination of semi-critical ultrasound probes
- NHSScotland Guidance for Decontamination and testing of Cardiac Heater Cooler Units (HCUs). v1.1 | National Services Scotland
Alternative approaches to decontamination
- Literature Review and Practice Recommendations: Existing and emerging technologies used for decontamination of the healthcare environment
Built environment
Addendum for High Consequence Infectious Disease (HCID)
1.0 About the HCID Addendum
This addendum contains information on HCID assessment PPE and associated donning and doffing procedures. It has been developed in collaboration with a task and finish group led by ARHAI Scotland. The sectors and networks represented can be found in Appendix 1.
A systematic literature review was undertaken by ARHAI Scotland to support the development of this addendum.
The HCID assessment PPE ensemble (defined in Section 2.0) has been endorsed by:
- the Health and Safety Executive (HSE)
- Advisory Committee on Dangerous Pathogens (ACDP)
- Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) Scotland
- NHS England
- Public Health Wales
- Public Health Agency Northern Ireland
- UK Health Security Agency (UKHSA)
2.0 Definitions contained within the HCID addendum
According to UK wide consensus (published by UKHSA), a HCID:
- is an acute infectious disease
- typically has high case-fatality rate
- may not have effective prophylaxis or treatment
- is often difficult to recognise and detect rapidly
- has the ability to spread in the community and within healthcare settings
- requires an enhanced individual, population, and system response to ensure it is managed effectively, efficiently, and safely.
The current list of HCIDs agreed by the UK 4 nations public health agencies, with advisory committee input as required is available.
The two types of UK HCID PPE ensemble used in the UK are:
- The HCID assessment PPE ensemble. This is the agreed unified UK PPE ensemble, which will be discussed in the sections below. It is worn by staff in NHSScotland to assess and provide clinical care to potential cases where HCID (associated with a pathogen of any transmission route) is suspected and may subsequently be confirmed prior to transfer to an HCID treatment centre in NHS England.
- The HCID treatment PPE ensemble. This is the ensemble worn for continued care of confirmed HCID cases within specialist treatment centres. There are currently none of these centres in Scotland.
The PPE ensemble described throughout this guidance is the ‘HCID assessment PPE ensemble’.
The healthcare worker roles described in this addendum are as follows:
- HCID caregiver or caregivers. This refers to the healthcare workers who will deliver direct care to a suspected or confirmed HCID case.
- Buddy. This refers to the healthcare worker who supports the HCID caregiver or givers during donning and doffing.
3.0 Scope and audience
It is expected that the HCID assessment PPE ensemble described in this addendum will be implemented by all NHSScotland Boards by 25 August 2026.
The HCID assessment PPE ensemble is intended for use when providing direct care for all suspected or confirmed cases of a HCID prior to transfer to a dedicated HCID treatment centre in NHS England.
Healthcare workers outside of HCID clinical pathways should aim to assess any suspected cases virtually for example by video or phone call. If a suspected or confirmed HCID case presents at a primary care facility, they should be isolated in a room away from all other healthcare workers and service users, and contact made with infectious disease services to arrange next steps.
It is important that NHS boards identify the most appropriate pathway locally, for the admission, care and transfer of a suspected or confirmed HCID case and ensure that staff within this pathway are trained in the use of the HCID assessment PPE ensemble. NHS boards should prepare to manage a suspected case admitted via any route including adult and paediatric EDs, or maternity units.
This guidance applies to healthcare workers and staff with responsibility for:
- care of an individual with a suspected or confirmed HCID
- the procurement, stock and storage of HCID assessment PPE ensemble and Buddy ensemble
- the fit testing of healthcare workers required to wear respiratory protective equipment (RPE) as part of the HCID assessment PPE ensemble or the Buddy ensemble
4.0 Personal Protective Equipment (PPE) for the care of individuals with suspected or confirmed HCID
4.1 HCID PPE Assessment ensemble and Buddy ensemble
4.1.3 Components of the HCID assessment PPE ensemble
Table 1: Components of the HCID assessment PPE Ensemble
Table 2: Components of the Buddy PPE Ensemble
4.1.4 Donning (putting on) HCID assessment PPE and Buddy ensemble
4.1.5 Demarcation of zones within HCID care area
Table 3: Zone Descriptors
4.1.6 Removal (doffing) of HCID assessment PPE and Buddy ensemble
Table 4: Activities permitted in each zone and doffing requirements for HCID Assessment PPE and Buddy ensemble
4.1 HCID PPE Assessment Ensemble and Buddy ensemble
In general, PPE ensembles should create a complete barrier at a sufficient level to protect against the risk of contamination with and transmission of infectious agents.
The key principle of the HCID assessment PPE ensemble is total body coverage, to minimise exposing skin or mucous membranes, and with filtering of inhaled air by a respirator. This ensemble has been developed to take account of the risk associated with the provision of direct care to a suspected or confirmed HCID case.
The buddy PPE ensemble has been developed to take account of the risks associated with the role of supporting the HCID caregiver to don and doff PPE where hands on assistance is essential.
The correct donning and doffing of the PPE ensembles is essential to ensure the PPE provides adequate protection against the risk of contamination and transmission of infectious agents.
Minimising contamination of PPE whilst in the red zone helps to reduce the consequences of any errors during doffing. See section 4.1.5 for techniques to avoid contamination.
4.1.1 Procurement and storage
It is important that departments identified for the care of individuals with a suspected or confirmed HCID, including emergency departments and intensive care units, have sufficient stocks of all HCID assessment PPE items described in this addendum.
Responsibility for the maintenance of adequate HCID PPE stock and appropriate storage should be assigned and managed locally to ensure prompt access in the event it is required.
The following points should be considered when procuring and storing HCID PPE items and is based on an estimate of 72 hours being the time taken to arrange transfer to an appropriate HCID treatment centre in NHS England. Some remote and rural boards may wish to hold a larger stock to take account of delivery times.
- A stock of PPE for education and training.
- Calculation of the number of PPE items required to care for an individual with a suspected or confirmed HCID over a period of at least 72 hours. The calculation should also consider the number of HCID caregivers expected to provide care to the suspected or confirmed HCID case and the number of supporting buddies, as well as an estimate of the number of PPE changes required throughout a shift.
- The need for availability of a variety of sizes of PPE to accommodate different healthcare workers.
- The need for respiratory protective equipment (RPE) for which healthcare workers have been fit-tested in advance.
- The need to regularly check stock availability and expiry dates.
Appendix 2 provides a list of the PPE components and specifications for purchasing. PPE products are available via National Procurement or independent suppliers where stated. These products are subject to change as and when national frameworks are awarded and the NDS supply and product code could vary depending on when orders are placed however the specification will remain unchanged.
4.1.2 Education and Training
It is important that NHS boards ensure the safety of all healthcare workers caring for patients with a suspected or confirmed HCID. All healthcare workers who may care for an individual with a suspected or confirmed HCID, and those who may undertake the buddy role, must be trained and competent in the donning, doffing and disposal of the HCID PPE ensemble described in this addendum.
Before using the ensemble described in this addendum, Healthcare workers must first complete the TURAS HCID Assessment PPE eLearning Module.
Healthcare workers should use the complementary resources below to reinforce their understanding of donning and doffing of the HCID assessment PPE ensemble and decontamination procedures for wellington boots.
- HCID assessment PPE donning training video
- HCID assessment PPE doffing training video
- HCID boot decontamination training video
- HCID Assessment PPE donning poster
- HCID Assessment PPE doffing poster
- HCID boot decontamination protocol (poster)
Healthcare workers should undertake donning and doffing practice sessions including verbal instructions from a buddy prior to undertaking a competency assessment.
Healthcare workers are considered competent in the donning and doffing of HCID assessment PPE when they can do so independently without prompts from the buddy. This ensures the healthcare worker can primarily demonstrate the order and process for donning and doffing without a verbal reminder. The inclusion of a verbal reminder during live clinical management of a suspected or confirmed case of HCID then acts as a further secondary level of assurance.
Competency checks should be undertaken at regular intervals to ensure competence is maintained. This should ideally be every 6 months but at least annually as a minimum.
Note: HCID PPE training does not include face fit testing. This must be done separately and it is assumed that all healthcare workers required to wear HCID PPE have already had face fit testing carried out. Information relating to face fit testing and fit checking can be found in the TBP chapter of the NIPCM.
Further training and educational resources for HCID are available from the UK HCID Network. NHS Boards may choose to utilise the ‘Train the trainer’ course to support the training of staff in their local board.
Boards are required to have a supply of the full HCID assessment PPE ensemble for training purposes which will include FFP3 masks of the correct type and size required for those identified as HCID caregivers and buddies. Training should be targeted at those identified as HCID caregivers and buddies.
A competency checklist to support training and education is available for use.
4.1.3 Components of the HCID assessment PPE ensemble
- All the components of this ensemble have been tested for suitability, ease of wear and safety benefits.
- All components of HCID PPE should conform to all relevant standards.
- See appendix 1 for links to published articles which describe the simulation exercise undertaken to support development of the HCID assessment PPE ensemble and a link to the literature review to support development of this addendum.
- Appendix 2 details PPE components for purchasing.
Table 1: Components of the HCID assessment PPE Ensemble
Component |
Requirement |
| FFP3 Respirator |
|
| Hood |
|
| Full-face visor |
|
| Gown |
|
| Apron |
|
| Inner gloves |
|
| Middle gloves |
|
| Outer gloves |
|
| Wellington Boots |
|
Table 2: Components of the Buddy PPE ensemble
(See table 4 for differences in Buddy PPE selection depending on HCID transmission type, room provision and zone access)
Component |
Requirement |
| Single pair of gloves |
|
| FFP3 Respirator |
|
| Fluid Resistant Surgical Mask (FRSM) |
|
| Full-face visor |
|
| Gown |
|
| Wellington Boots |
|
4.1.4 Donning (putting on) HCID assessment PPE and Buddy ensemble
Prior to donning the HCID assessment PPE ensemble and caring for a patient with a suspected HCID, the HCID caregiver should ensure that they feel well, are well hydrated and have been to the toilet.
Change into surgical scrubs (if not already wearing). Long hair must be tied back, and all jewellery and ID badges or lanyards removed.
A trained buddy should provide verbal assistance and visual cues to instruct the HCID caregiver in putting on (donning) the PPE and must perform a final visual inspection of the ensemble. The buddy must remain in the green zone at all times when providing verbal instruction.
Before entering the patient care area, a check of the doffing area should be performed to ensure all necessary supplies, for example waste receptacle and hand rub, for doffing are available and the zones of the doffing area are demarcated correctly. See section 4.1.5 for Demarcation of zones within HCID care area.
An illustrated step-by-step guide to putting on the individual components of the HCID assessment PPE ensemble is available. Copies of illustrated guidance should be displayed in the area where PPE is put on. It is important that guidance is checked regularly to ensure the most recent version is displayed.
Buddies must don PPE and enter the amber zone only if essential for example, if the caregiver requires assistance with PPE removal. Buddy PPE should be donned in the following order:
- wellington boots
- disposable, fluid-resistant gown
- FRSM/FFP3 respirator
- full-face visor
- non-sterile gloves worn over gown cuffs
4.1.5 Demarcation of zones within HCID care area
It is important to identify zones in the care area designated for care of a suspected or confirmed HCID. The zone descriptors and associated permitted activity outlined in table 3 and 4 have been developed to help aid local boards in developing zones and denote risk determined by the activities undertaken within them and the potential exposure to blood, body fluids or aerosols associated with the suspected or confirmed HCID case. Tape may be used on the floor to demarcate zones.
Table 3: Zone Descriptors
Zone |
Description |
| Red | An area where there is a risk to healthcare workers of direct exposure to a patient suspected or confirmed with HCID, for example within the patient room where direct care is provided. Doors to the red and amber area should remain shut during care provision and at all times other than on entry and exit. |
| Amber | An area situated in the vicinity of the individual with suspected or confirmed HCID* which therefore poses a potential threat of indirect exposure to healthcare workers from infectious particles in the environment, on equipment during doffing of PPE or from doffed and discarded PPE (waste), for example an ante room or an area demarcated as the amber zone outside the isolation room. *The optimum placement for a patient with a suspected or confirmed airborne HCID is a negatively pressured room with a negatively pressured ante room (amber zone). The pressure cascade in a negatively pressured room should be ante room to patient room or in the absence of an ante room, corridor to patient room. Positive pressure ventilated lobby (PPVL) rooms are not recommended for use for the care of a HCID patient due to the risk of air leakage from the ante room to the corridor. However, where a PPVL room is available, this may provide greater protection than a standard single room without specialised ventilation. There is a risk of infectious particles leaking into the corridor in the following room provisions:
It is not possible to quantify this risk and more research is required. |
| Green | An area demarcated as the green zone and separated from the individual with suspected or confirmed HCID by a physical barrier, where no clinical care or doffing practices take place and where no contaminated equipment and/or waste associated with the suspected or confirmed HCID case is present therefore posing no threat of HCID exposure to healthcare workers. |
Within the Scottish Ambulance Service (SAS), the patient saloon within the ambulance and any area in which the patient is being treated, for example patients own home, would be considered the red zone. SAS staff should identify a suitable area for donning and doffing of PPE.
Minimising contamination of PPE in the red zone helps to reduce the consequences of any errors during doffing. The following techniques may be employed.
- Maintain distance from the patient where possible.
- Avoid touching or leaning on objects and surfaces where possible (consider adopting a 'clasped hands' position).
- Consider changing outer pair of gloves between care events.
4.1.6 Removal (doffing) of HCID assessment PPE and Buddy ensemble
An illustrated step-by-step guide to removing the individual HCID assessment PPE components is available. Copies of illustrated guidance should be displayed in the PPE removal area, but this does not replace the need for a buddy. It is important that illustrated copies displayed are checked regularly to ensure the most recent version is displayed.
A buddy system is essential for the entire PPE removal process (doffing) to ensure that this is performed safely. A buddy who is trained and competent in the use of this PPE should assist the HCID caregiver by providing verbal instructions, visual cues, and reminders as necessary.
The HCID caregiver removing PPE and the buddy must aim to observe a strict no-touch policy and maintain a separation of at least 1 metre during the PPE removal process. The buddy must only intervene where the HCID caregiver is having a problem removing the ensemble safely. In this case buddies must don appropriate PPE before entering the amber zone to provide assistance.
If a buddy is required to wear PPE (see table 3 for PPE requirements), it should be doffed using the techniques for each item described in appendix 6 and in the following order:
- gloves
- gown
- full-face visor
- FRSM/FFP3
- Wellington boots
Guidance for doffing of boots by buddies and HCID care givers is the same.
Hand rub should be used after doffing gloves and again after doffing gown. Perform hand hygiene once all PPE is removed.
Demarcation zones described above are necessary to ensure all HCID care givers and buddies remain safe during the doffing process. Table 4 sets out the activities permitted and PPE requirements and instructions for both the HCID care giver and the buddy determined by each zone.
All doffed PPE including boots should be managed as per section 5.
Table 4: Activities permitted in each zone and doffing requirements for HCID Assessment PPE and Buddy ensemble
Zone |
HCID caregiver activity and PPE requirements and instructions |
Buddy PPE requirements and instructions |
| Red | Direct care provision Full HCID assessment PPE ensemble must be worn at all times within the red zone. Practice contamination reduction measures where possible. When ready to leave care area remove the following PPE items in this zone only:
|
Must not enter this zone at any time. |
|
Amber (See also optimum patient placement details in table 3) |
To be used for doffing and PPE disposal on exit from patient room. Remove the remainder of PPE items in this zone. HCID care giver must not enter green zone until all PPE has been doffed. |
Airborne HCID – patient room and ante room negatively pressurised
The buddy must not enter the green zone until all PPE has been doffed. |
|
Airborne HCID – either patient room and/or ante room are NOT negatively pressurised
The buddy must not enter the green zone until all PPE has been doffed. |
||
| Contact HCID Must only enter this zone to assist HCID care giver or decontaminate equipment. Will require the following PPE:
|
||
| Green | No care is provided in this area. All PPE items must have been doffed (removed) in the amber area. Before leaving this zone, HCID care giver may reach into the amber zone to place boots into the isolation bin. Immediately move to a clinical hand washing basin after doffing to undertake hand hygiene using liquid soap and water. |
Buddy providing verbal instruction only to HCID care giver. No contact with HCID caregiver or equipment. If the buddy has doffed PPE, before leaving this zone, the buddy may reach into the amber zone to place boots into the isolation bin. Immediately move to a clinical hand washing basin to undertake hand hygiene using liquid soap and water. |
5.0 Management and disposal of used PPE after doffing
A large amount of waste may be generated when caring for suspected or confirmed cases of HCID. It is therefore important to seek advice from and alert the local waste management team at the earliest opportunity to enable the initiation of local waste protocols for HCID.
All components of the HCID assessment PPE and buddy PPE ensemble should be single-use disposable, except for wellington boots. Boots should be quarantined in a designated container until the HCID result is known and action taken as outlined in table 5. Wellington boots must only be re-used if the case is HCID negative. Guidance for the decontamination of wellington boots is available should the patient subsequently test HCID negative.
Note: In NHSScotland, sporicidal wipes are not commonly used and a chlorine based solution of 1000ppm should be used to decontaminate the boots where there is no visible soiling and patient case is confirmed HCID negative.
Table 5 - PPE Waste Disposal and Wellington Boot Management
HCID case |
PPE Waste Disposal |
Wellington Boot Management |
| Confirmed | PPE waste including reusable PPE should be held in a secure location and disposed of as Category A waste as per SHTN 03-01. | Wellington boots should be held securely along with other PPE waste in a secure location and disposed of as Category A waste. |
| Suspected | If results are positive treat PPE waste as for a confirmed case, see above. If results are negative the waste can be disposed of as clinical waste. |
If results are positive treat Wellington boots as for a confirmed case, see above. If results are negative wellington boots can be decontaminated. See guidance above. |
6.0 Exposure safety and PPE Breach
To ensure the safety of all individuals it is vital that:
- there is provision of HCID assessment PPE and buddy PPE training which includes exposure awareness and clear guidance on the immediate management of any sharp’s injuries or exposures or PPE breaches
- there is ongoing monitoring of competence
- all the instructions regarding donning, doffing, and checks at every stage in the process are followed carefully
- a register of all Healthcare workers entering the patient's room is kept for a period of time relevant to the incubation period of the pathogen or disease as per local board procedures
- practice contamination reduction measures described in section 4.1.5 where possible
- any exposures or injury are recorded as per local board reporting or as required by HSE
More information on exposure safety and VHF is available.
Appendix 1: Key reading to support the UK HCID assessment PPE ensemble
The HCID assessment PPE ensemble, including specifications, and donning and doffing protocols in this guidance are based on evidence provided by simulation experiments undertaken by HSE in conjunction with academic institutions and NHS providers.
- Hall S, Poller B, Bailey C, et al. Use of ultraviolet-fluorescence-based simulation in evaluation of personal protective equipment worn for first assessment and care of a patient with suspected high-consequence infectious disease. J Hosp Infect. 2018; 99: 218-228.
- Poller B, Hall S, Bailey C, et al. ‘VIOLET’: a fluorescence-based simulation exercise for training healthcare workers in the use of personal protective equipment. J Hosp Infect. 2018; 99(2): 229-235.
- Poller B, Tunbridge A, Hall S, et al. A unified personal protective equipment ensemble for clinical response to possible high consequence infectious diseases: A consensus document on behalf of the HCID programme. J Infect. 2018; 77(6): 496-502.
A systematic literature review was undertaken to support the development of this addendum. Recommendations, Good Practice Points and the final addendum content were developed by a dedicated task and finish group with representation from the following sectors and networks:
- Infectious diseases services
- Maternity services
- Critical care services
- Emergency department services
- National Procurement
- Scottish Ambulance Service
- Health and Safety
- Infection Control Managers Network
- Infection Control Doctors Network
- Senior Infection Prevention and Control Nurses Network
- Primary care services
- NHS Education for Scotland
Appendix 2: HCID Assessment PPE components and specifications for purchasing
Item |
NHSScotland Component Specification |
| FFP3 Respirator |
|
| Hood* |
*Health boards can purchase directly from SCCL via the online catalogue. |
| Full-face visor |
|
| Gown |
|
| Apron |
|
| Inner gloves |
|
| Middle gloves |
|
| Outer gloves |
|
| Wellington Boots |
|
Note. Product codes are correct at the time of publishing, however, they are subject to change.
Any changes will be managed by the NDS supply chain Pecos content manager (PCM).
All products, apart from the hoods, are available to order via PECOS. Hoods can be ordered directly from the supply chain.
Process for ordering
Those responsible for ordering the PPE should access the HCID PPE PPE catalogue within PCM.
All items for ordering will be visible there with the current SKU as displayed in the catalogue. The items can then be ordered via Pecos.
Any queries should be directed to the national procurement team at nss.ppecell@nhs.scot
Care Home Infection Prevention and Control Manual (CH IPCM)
When an organisation uses products or adopts practices that differ from those stated in this Care Home Infection Prevention and Control Manual, that individual organisation is responsible for ensuring safe systems of work including the completion of risk assessments approved through local governance procedures.
Use of this manual online is advised as printed versions are uncontrolled.
The ARHAI Scotland Transmission Based Precautions (TBPs) literature review is currently ongoing and so the information may be subject to change.
 View latest news and updates for the NIPCM and CHIPCM
View latest news and updates for the NIPCM and CHIPCM
Last updated: 21 August 2024
What is the Care Home Infection Prevention and Control Manual (CH IPCM)?
The Care Home Infection Prevention and Control Manual (CH IPCM), referred to as ‘the manual’ throughout, was first published in 2021. It is evidence-based and is intended to be used by all those involved in care home provision in Scotland.
The manual is context specific and has been co-produced with national and local stakeholders. The content of the manual is completely aligned to the evidence based National Infection Prevention and Control Manual (NIPCM) which was first published in 2012, by the Chief Nursing Officer (CNO (2012)1).
The manual currently contains
- Chapter 1 – Standard Infection Control Precautions (SICPs)
- Chapter 2 – Transmission Based Precautions (TBPs)
The manual is a practice guide for use in care homes. When used, it can help reduce the risk of infections and ensure the safety of residents, as well as staff and visitors in the care home environment. It is the Scottish Government expectation that care home settings apply guidance contained within this manual to achieve the aims.
The manual aims to:
- make it easy for staff to apply effective infection prevention and control (IPC) precautions
- help reduce the risk of infection
- reduce variation, promote standardisation, and optimise IPC practices throughout care home settings
- improve the application of staff knowledge and skills in IPC
- help align practice, monitoring, quality improvement and scrutiny
The manual should be adopted for all IPC practices and procedures within care home settings.
Is the manual based on scientific literature?
The recommendations for practice made in the manual are fully aligned to the NIPCM and are based on real-time reviews of the current scientific literature (for example Medical Journals) and best practice. Any major changes identified in the scientific literature may lead to a change being made to the content, and so, it is recommended that the online version is always accessed and used locally.
What's in the appendices?
The appendices can be used as practical implementation of the manual and contain graphical representations (for example diagrams and charts) that can be used along with the contents of the manual.
Many of the appendices can be printed off as posters for local use throughout the care home.
Are there any other IPC materials that can be used?
There are links throughout the manual to additional resources and the resources page links to IPC campaign materials, education, training links and posters.
In addition you may find it useful to read the literature reviews and SBARs for the manual and the A-Z of pathogens.
How can I find out what the scientific and medical words mean?
A glossary section has been provided.
Does the manual content work on mobile devices?
Yes, all content including appendices work on mobile devices for example laptops and smartphones.
Responsibilities for the CH IPCM
Responsibilities for content of the manual
ARHAI Scotland to ensure:
- that the content of this manual remains evidence based or where evidence is lacking, content is based on consensus of expert opinion
Stakeholders of ARHAI Scotland programme working groups to ensure:
- full participation in the working groups including full engagement with the consultation process outlined in the Terms of Reference associated with each working group
Responsibilities for the adoption and implementation of this manual
The manual should be used by:
- care home organisations
- care home staff including permanent, agency and where required external contractors
- health protection teams
- infection prevention and control teams
- professionals providing support
- individuals visiting the care home
Care home providers to ensure:
- the adoption and implementation of this manual in accordance with existing local governance processes
- that systems and resources are in place to facilitate implementation and compliance monitoring of IPC as specified in this manual in all care areas
- compliance monitoring includes all staff (permanent, agency and where required external contractors)
- there is an organisational culture which promotes incident reporting and focuses on improving systemic failures that encourage safe IPC working practices including near misses
- there is a nominated lead with responsibility for IPC within the care home
Care home managers to ensure that all staff:
- are aware of and have access to this manual
- have completed appropriate IPC training relevant to their roles and that this is centrally recorded. Training may include resources developed by your organisation, your local NHS board, Health and Social Care Partnership, NHS Education for Scotland (NES) or the Scottish Social Services Council (SSSC)
- have adequate support and resources available to enable them to implement, monitor and take corrective action to ensure full compliance with this manual
- implement a robust risk assessment including detailing any deviations from the manual and the local mitigation measures that were undertaken and post-approval documented via local governance procedures
- with health concerns (including pregnancy) or those who have had an occupational exposure relating to IPC are timeously referred to the relevant agency, for example general practitioner (GP or doctor), occupational health or if required accident and emergency
- have undergone the required health checks or clearance (including those undertaking exposure prone procedures (EPPs))
- include IPC as an objective in their personal development plans (or equivalent)
Care home staff providing care to:
- understand, adopt and implement the principles of IPC as set out in this manual
- maintain IPC competence, skills, and knowledge through completing appropriate training relevant to their role as directed by their line manager. IPC training, NHS Education for Scotland (NES) or the Scottish Social Services Council (SSSC)
- communicate the IPC practices to be taken to appropriate colleagues, residents, relatives and visitors without breaching confidentiality
- have up to date occupational immunisations/health checks/clearance requirements as appropriate
- report to line managers and document any gaps in IPC knowledge, resources, equipment and facilities or incidents that may result in transmission of infection including near misses for example sharps or PPE failures
- do not provide care while at risk of potentially transmitting infectious agents to others - if in any doubt they should consult with their line manager, occupational health department, local infection prevention and control team (IPCT) or local health protection team (HPT)
- contact the local HPT/IPCT if there is a suspected or actual HAI incident/outbreak
Local infection prevention and control teams (IPCTs) and health protection teams (HPTs) to:
- engage with care home staff to develop systems and processes that lead to sustainable and reliable improvements in relation to the application of IPC practice
- provide expert advice on the application of IPC and provide support when requested to develop individual or organisational risk assessments where deviations from the manual are necessary
- have epidemiological or surveillance systems capable of distinguishing resident case or cases requiring investigations and control
- provide local support and advice (when necessary and/or requested) and complete documentation when an incident/outbreak or data exceedance is reported
Chain of infection
In order for infection to occur several things have to happen. This is often referred to as the chain of infection. The six links in the chain are:
- infectious agent or the microorganism which can cause disease
- reservoir or source of infection where the microorganism can live and thrive. This may be a person, an animal, any object in the general environment, food or water
- portal of exit from the reservoir. This describes the way the microorganism leaves the reservoir. For example, in the case of a person with flu, this would include coughing and sneezing. In the case of someone with gastroenteritis microorganisms would be transmitted in the faeces or vomit
- mode of transmission. This describes how microorganisms are transmitted from one person or place to another. This could be via someone’s hands, on an object, through the air or bodily fluid contact
- portal of entry. This is how the infection enters another individual. This could be landing on a mucous membrane, being breathed in, entering via a wound, or a tube such as a catheter.
- susceptible host. This describes the person who is vulnerable to infection.
Infection can be prevented by breaking the chain of infection.
Chain of infection diagram
The overall aim of Standard Infection Control Precautions (SICPs), is to break the chain of infection.
The chain of infection diagram illustrates and gives examples of actions that can be taken to break it.
Select image for full size version.
![]() Use the NES SIPCEP Breaking the Chain of Infection module to learn about breaking the chain of infection in care homes.
Use the NES SIPCEP Breaking the Chain of Infection module to learn about breaking the chain of infection in care homes.
Hierarchy of controls
Reducing risk
The hierarchy of controls (HoC) is a system used to help prevent the transmission of infection. It details the most to least effective controls. You will note that PPE is the last level of control in the hierarchy, used when all other controls have not reduced the risks sufficiently. To be effective, PPE must be used correctly which means putting it on and removing it correctly and safely.
![]() See the Health and Safety Executive’s (HSE) toolkit on managing risks and risk assessment at work.
See the Health and Safety Executive’s (HSE) toolkit on managing risks and risk assessment at work.
The HoC principles can be broadly interpreted for care home settings and include:
- reducing hazards in the care home
- changing practice in the care home
- making the care home as safe as possible
- changing how we organise and work in the care home
- use of PPE
Examples of HoC principles
Here are some examples of how to apply the HoC principles in care home settings. These examples do not cover every situation where you might need to use HoC principles.
Reducing hazards in the care home
Measures such as vaccination, testing and isolation help to reduce the risk of infection. Not coming to work when ill, isolating while infectious and recognising and reporting infections promptly, all help to prevent infections spreading.
Changing practice in the care home
When faced with a particular risk, such as an outbreak, we may need to change what we do. This might include reducing communal activities, considering limiting visiting for a short period of time, or cleaning the care home environment more frequently. The local IPCT and/or HPT should always be contacted for advice and support in outbreak situations.
Making the care home as safe as possible
It is very unlikely that we will be able to change where we work but the care home setting should be made as safe as possible.
You can reduce opportunities for pathogens to survive in the care home by ensuring fixtures and fittings are in good repair and can be easily cleaned and following water safety guidelines.
Ventilation is also an effective measure to reduce the risk of some respiratory infections, by diluting and dispersing the pathogens which cause them. Consider opening windows and vents more than usual, even opening a small amount can be beneficial. Opening windows and doors may present security and safety issues and so a local risk assessment should always be undertaken.
Changing how we organise and work in the care home
Changing the way, we organise and work in the care home can also help reduce risk. This might include reducing the number of people in a space at any one time and minimising the movement of staff between different settings as well as using administrative controls.
Administrative controls include local risk assessments, staff training, IPC audits, and providing clear signage and instructions throughout the care home.
Chapter 1: Standard Infection Control Precautions (SICPs)
The basic IPC measures that should be used in your care home are called Standard Infection Control Precautions (SICPs).
SICPs are used to reduce the risk of transmission of infectious agents from known and unknown sources of infection.
These should be used by all staff, in all care settings, at all times, for all residents whether infection is known to be present or not to ensure the safety of residents, staff and visitors in the care home.
SICPs should be part of everyday practice and applied consistently by everyone in the care home.
It is essential that optimal IPC measures are applied continuously as residents living in care homes are more vulnerable, therefore increasing their risk of acquiring infections which may then be serious and potentially life threatening. By applying optimum IPC precautions you will provide a safe environment and effective care.
There are 10 Standard Infection Control Precautions (SICPs)
- resident placement/assessment for infection risk
- hand hygiene
- respiratory and cough hygiene
- personal protective equipment
- safe management of care equipment
- safe management of care environment
- safe management of linen
- safe management of blood and body fluid spillage
- safe disposal of waste
- occupational safety: prevention and exposure management (including sharps)
1. Resident placement/assessment for infection risk
Before a resident is admitted to the care home it is important to risk assess for infection as part of resident’s care plan, an IPC admission assessment should be undertaken by staff.
If you suspect or are aware that a resident has an infection, then details should be confirmed for the correct IPC precautions to be put in place for the safety of the resident and others.
Obtaining infection details may include appropriate clinical samples and/or screening to establish the causative organism which may be on advice from your local GP, IPCT or HPT.
Residents who may present a cross-infection risk include those with:
- diarrhoea
- vomiting, being sick
- unexplained rash
- fever or temperature of 37.8 C or higher
- respiratory symptoms such as coughing and sneezing
- known to have been previously positive with a Multi-drug Resistant Organisms (MDRO), for example Meticillin Resistant Staphylococcus aureus (MRSA), Carbapenemase Producing Enterobacterales (CPE)
Further information regarding general respiratory screening questions can be found within the resources section of the NIPCM.
Note: If a resident requires isolation because of infection or in an outbreak situation, this should be individually risk assessed to ensure the safety and health and wellbeing needs of the resident. Isolation periods must be monitored on daily basis and be for the minimum period specified.
Resources
![]() Appendix 11 of the NIPCM gives you further information on the precautions required for different infections.
Appendix 11 of the NIPCM gives you further information on the precautions required for different infections.
 Read the placement literature review to understand the evidence base for resident placement.
Read the placement literature review to understand the evidence base for resident placement.
2. Hand hygiene
Please note that the term ‘alcohol-based hand rub (ABHR)’ has now been updated to ‘hand rub’. A hand rub (alcohol or non-alcohol based) can be used if it meets the required standards. Please see further information in the hand hygiene products literature review.
The most important thing you can do to prevent the spread of infection in a care home is to keep your hands clean. This is called hand hygiene.
Adherence with the following points is essential to ensure effective hand hygiene:
- expose forearms (known as bare below the elbows)
- remove all hand/wrist jewellery including any embedded jewellery (a single, plain metal finger ring or ring dosimeter (radiation ring) is permitted but should be removed (or manipulated) during hand hygiene). Bracelets or bangles such as the Kara which are worn for religious reasons should be able to be pushed higher up the arm and secured in place to enable effective hand hygiene which includes the wrists
- ensure fingernails are clean, short and that artificial nails or nail products are not worn
- cover all cuts or abrasions with a waterproof dressing
Hand washing should be extended to the forearms if there has been exposure of forearms to blood and/or body fluids.
Hand washing sinks should only be used for hand hygiene and should not be used for the disposal of other liquids.
To perform hand hygiene:
- hand rub should be available for staff as near to point of resident care as possible. Where this is not practical, personal hand rub dispensers should be used
- application of sufficient volume of hand rub to cover all surfaces of the hands is important to ensure effective hand hygiene
- manufacturer’s instructions should be followed for the volume of hand rub required to provide adequate coverage for the hands. In the absence of manufacturer’s instructions, volumes of approximately 3ml are recommended to ensure full coverage
- wall mounted or personal hand rub dispensers (cartridges/bottles) should not be refilled and should be replaced when empty or if outwith manufacturer expiry date
The World Health Organization’s ‘4 moments for hand hygiene’ should be used to highlight the key indications for hand hygiene:
- before touching a resident
- before clean/aseptic procedures. If hand rub cannot be used, then antimicrobial liquid soap should be used
- after body fluid exposure risk
- after touching a resident
Some additional examples of hand hygiene moments include, but are not limited to:
- after touching a resident’s immediate surroundings
- before handling medication
- before preparing food
- before donning (putting on) and after doffing (taking off) PPE
- after visiting the toilet
- before putting on and after removing PPE
- between carrying out different care activities on the same resident
- after cleaning and disinfection procedures
- after handling used linen
- after handling waste
It is important that residents are routinely encouraged to perform hand hygiene and given assistance if required.

The four moments for hand hygiene poster can be used in your care home to show staff when hand hygiene should be done and the reasons why.
Select image for full size version.

Hands should be washed with liquid soap and water if/when:
- they are visibly soiled or dirty
- they are potentially contaminated with blood, other body fluids or excretions
- caring for a resident with vomiting or diarrhoeal illness
- caring for a resident with a suspected or known gastro-intestinal infection such as norovirus or a spore forming organism such as Clostridioides difficile
Note:
Hands should be washed with warm/tepid water to mitigate the risk of dermatitis associated with repeated exposures to hot water and to maximise hand washing compliance. Compliance may be compromised where water is too hot or too cold.
Hands should be dried thoroughly following hand washing using a soft, absorbent, disposable paper towel from a dispenser which is located close to the sink but beyond the risk of splash contamination.
The use of antimicrobial hand wipes is only permitted where there is no access to running water. Staff should perform hand hygiene using hand rub immediately after using the hand wipes and perform hand hygiene with soap and water at the first available opportunity.
In all other circumstances use hand rub for routine hand hygiene.
Skin care
- Hand rubs should contain emollients in their formulation.
- Pat hands dry after hand washing using disposable paper towels. Avoid rubbing which may lead to skin irritation/damage.
- Use an emollient hand cream during breaks and when off duty. These should be applied all over the hands including between the fingers and the back of the hands.
- Staff with skin problems should seek advice from the local occupational health department if available or their GP.
- Barrier creams should not be used in the workplace.
Do not use refillable containers or communal tubs of hand cream in the care home setting.
Resources

Read the hand hygiene literature reviews to find out more about the evidence base for hand hygiene.
 To make sure you clean your hands properly with soap and water you should follow the steps in the poster ‘How to hand wash step by step images’. This poster can be printed off and displayed throughout the care home to ensure that all staff and visitors are aware of and practice this hand hygiene method when required in the care home.
To make sure you clean your hands properly with soap and water you should follow the steps in the poster ‘How to hand wash step by step images’. This poster can be printed off and displayed throughout the care home to ensure that all staff and visitors are aware of and practice this hand hygiene method when required in the care home.
Select image for full size version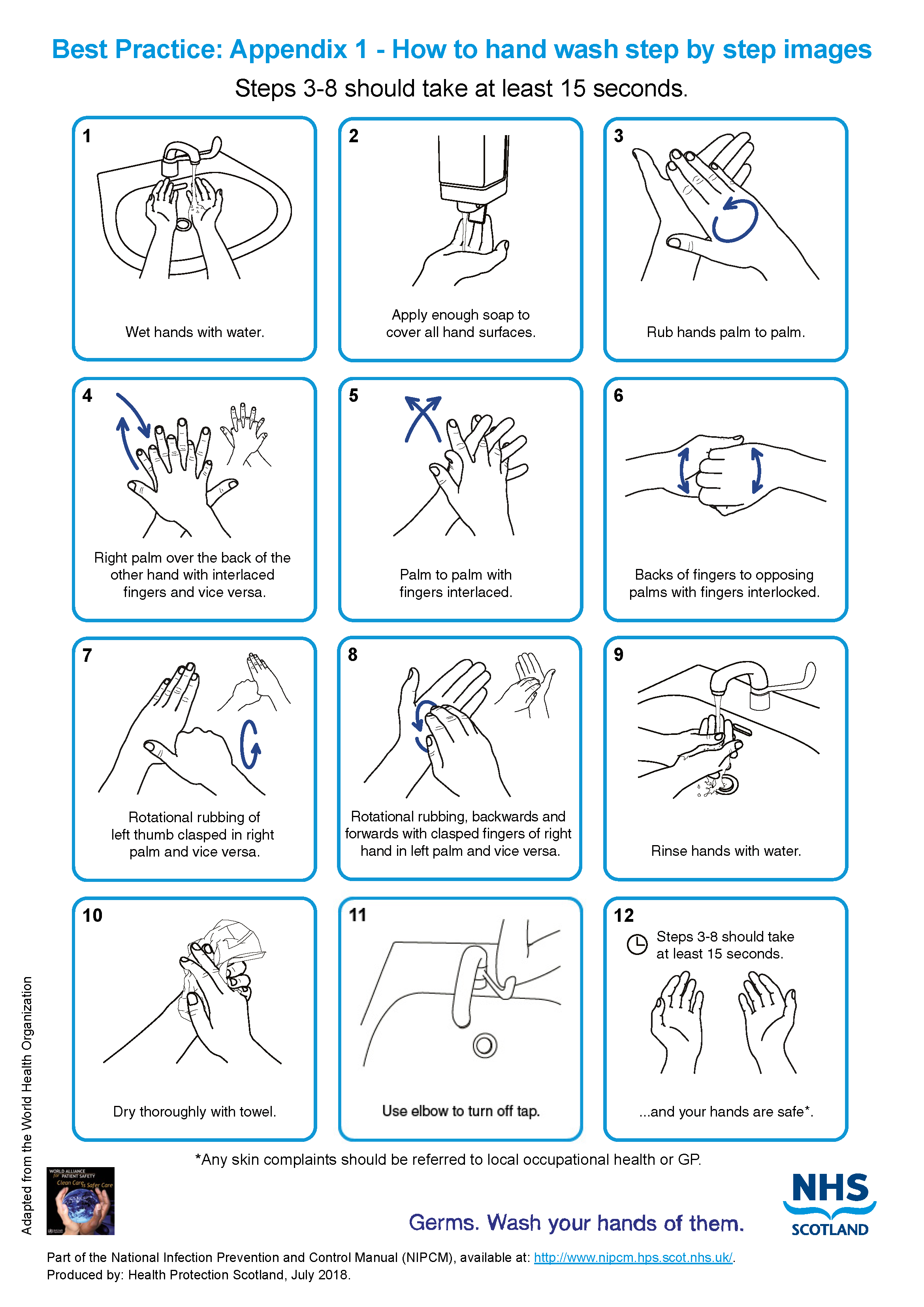
 To make sure you clean your hands properly with hand rub you should follow the steps in the poster ‘How to hand rub step by step images’. This poster can be printed off and displayed throughout the care home to ensure that all staff and visitors are aware of and practice this hand hygiene method when required in the care home.
To make sure you clean your hands properly with hand rub you should follow the steps in the poster ‘How to hand rub step by step images’. This poster can be printed off and displayed throughout the care home to ensure that all staff and visitors are aware of and practice this hand hygiene method when required in the care home.
Select image for full size version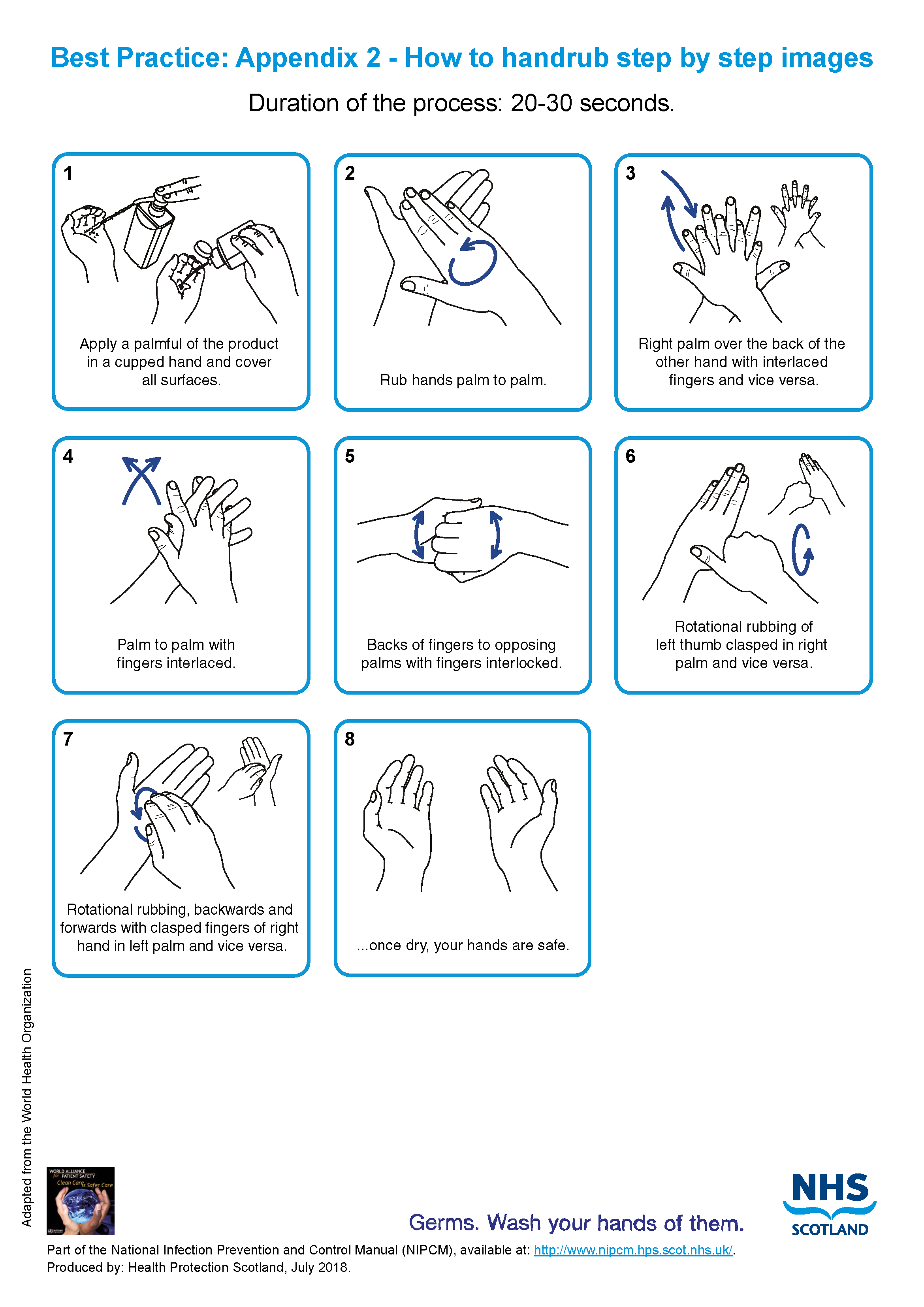
3. Respiratory and cough hygiene
Infections can spread by coughing and sneezing, therefore it is very important that respiratory and cough hygiene is used by everyone including staff, residents and visitors.
Any resident displaying symptoms of respiratory illness should be encouraged to wear a surgical (for instance TYPE IIR FRSM) face mask where it is clinically safe and can be tolerated by the wearer, especially in communal areas.
What you need for respiratory and cough hygiene• disposable tissues• waste bin and waste bags• hand hygiene products
If a resident has a cough, cold or other respiratory symptoms then they should be supported and encouraged to:
- cover their nose and mouth with a disposable tissue when sneezing, coughing, wiping and/or blowing the nose. If a disposable tissue is not available encourage the use of elbow/sleeve to cover the nose and mouth when coughing or sneezing
- put used tissues and face masks into a waste bin immediately after use
- wash their hands with soap and water after coughing, sneezing, using tissues, or after contact with respiratory secretions or objects contaminated by these secretions
- keep contaminated hands away from the eyes nose and mouth
Staff should:
- promote respiratory and cough hygiene and help residents who need assistance
- ensure that residents are provided with the necessary products for respiratory and cough hygiene including tissues, waste bag and hand hygiene products and make sure that products are in close vicinity for the resident to access
- use hand wipes followed by hand rub if there is no running water available or if hand hygiene facilities are out of reach then wash hands at the first available opportunity.
 Read the respiratory and cough hygiene literature review to find out the evidence for respiratory and cough hygiene practice.
Read the respiratory and cough hygiene literature review to find out the evidence for respiratory and cough hygiene practice.
4. Personal Protective Equipment (PPE)
Disposable items of PPE you might require at the point of care delivery. • gloves• aprons• gown• masks• eye/face protection
Deciding which PPE to use
Before doing any procedure or task staff should risk assess any likely exposure to blood and/or body fluids and ensure PPE is worn that provides adequate protection against the risks associated with the procedure or task being undertaken.
All PPE should be:
- located close to the point of use
- stored in a clean and dry area to prevent contamination until needed for use
- within expiry dates
- single-use only items unless specified by the manufacturer
- changed immediately after individual use and/or following completion of a procedure or task
- disposed of after use into the correct waste stream as per local risk assessed processes
Reusable PPE items, for example non-disposable goggles, face shields or visors must be cleaned/decontaminated once removed or placed within a designated container for subsequent cleaning/decontamination with decontamination schedules in place and responsibility assigned.
Gowns, headwear and footwear are unlikely to be required within a care home setting. Please refer to the NIPCM for guidance on use.

Read further information on best practice for PPE in Appendix 15.
Donning (putting on) personal protective equipment (PPE)
The order for putting on PPE is:
- apron or gown
- surgical mask
- eye/face protection (where required)
- gloves
Doffing (taking off) personal protective equipment (PPE)
It is important that PPE is removed in the correct order.
The order for taking off PPE is:
- gloves
- apron or gown
- eye/face protection (if worn)
- surgical mask
Note:
Always carry out hand hygiene immediately after taking off PPE.
If using eye and face protection hand hygiene should be carried out after removing the apron or gown and before removing eye and face protection.
All PPE should be removed removed, followed by hand hygiene, before leaving the area and disposed of as healthcare waste.
Resources
 A poster showing the order for putting on and removing PPE is available to print.
A poster showing the order for putting on and removing PPE is available to print.
Select image for full size version
Gloves
Gloves should be:
- worn when it is likely or anticipated that you will be exposed to blood, body fluids (including but not limited to secretions and/or excretions), non-intact skin, mucous membranes, lesions and/or vesicles, hazardous drugs, and chemicals for example cleaning agents
- single-use and should be donned (put on) immediately prior to exposure risk and should be doffed (taken off) immediately after each use or upon completion of a task
- appropriate for use, fit for purpose and well-fitting. The glove selection chart can help you select the correct gloves
- changed if damaged or a perforation or puncture is suspected
Note:
Using gloves reduces the risk of contamination but does not remove all risk.
Gloves should not be used instead of carrying out hand hygiene.
Gloves should not be worn inappropriately in situations such as to go between residents, move around a care area or whilst at workstations (on the telephone or computer).
Gloves should never be decontaminated or cleaned with hand rub or by washing with cleaning products.
![]() Use the glove selection chart to support you to select the correct glove type.
Use the glove selection chart to support you to select the correct glove type.
Select image for full size version
Aprons and gowns
Selection of aprons or gowns for use in health and care settings should be based on an assessment of the task to be undertaken, and the anticipated levels of blood or body fluid exposure.
Aprons should be:
- disposable
- fluid repellent
- used to protect the uniform, workwear or clothes to prevent exposure or contact with another person’s:
- blood or bodily fluids (including urine, faeces, vomit, nasal discharge, wounds, sores)
- mucous membranes (including nose and mouth)
- rashes (confirmed or suspected scabies)
- leaking vesicles (confirmed or suspected shingles)
- used to protect uniform, workwear or clothes when exposure or contact with hazardous drugs, chemical or cleaning products is likely
- used to protect uniform, workwear or clothes when contact or exposure or handling of used, soiled, or infectious linen /laundry is likely
- to protect uniform, workwear or clothes when providing direct care to a resident during assisted toileting, or eating
- regarded as single use items which should never be reused (one resident, one task, one apron)
Eye/face protection
Eye/face protection should:
- be worn when there is an anticipated risk of splashing or spraying of blood or bodily fluids and always during Aerosol Generating Procedures (AGPs)
Note:
Eye/face protection should not be touched when worn or worn around the neck or on top of the head when not in use.
Eye/face protection should be compatible with other items of PPE and worn in accordance with manufacturer’s instructions.
Prescription eyeglasses and contact lenses should not be considered a form of eye/face protection.
Fluid Resistant Type IIR surgical face masks (FRSM)
Fluid Resistant Type IIR surgical face masks should be:
- worn if splashing or spraying of blood, body fluids, secretions or excretions onto the respiratory mucosa (nose and mouth) is expected/likely. (As part of SICPs a full-face visor may be used as an alternative to fluid resistant Type IIR surgical face masks to protect against splash or spray)
- well-fitting, fully covering the mouth and nose and fit for purpose. Manufacturer’s instructions should be followed to ensure effective fit/protection
- removed or changed:
- at the end of a procedure/task
- if the mask is damaged or there is a build up from moisture after prolonged use or from gross contamination with blood or body fluids
- following specific manufacturers’ instructions
If you are using droplet precautions, you should always wear a Type IIR surgical face mask as well as eye/face protection (droplet precautions will be discussed further in Chapter 2 Transmission Based Precautions).
Transparent face masks
Transparent face masks may be used to aid communication with residents where required.
Transparent face masks should:
- meet the specification standards of the Transparent face mask technical specification
and
- have been approved for use within health and social care settings
- only be worn in areas where Fluid Resistant Type IIR surgical face masks are used as personal protective equipment
Resources
 Read the aerosol generating procedures literature review and surgical face masks literature review for further information regarding the evidence base.
Read the aerosol generating procedures literature review and surgical face masks literature review for further information regarding the evidence base.
See appendix 11 for further information.
PPE for visitors
Where visitors are undertaking any direct care activities which may result in them encountering blood or other bodily fluids, they should also be offered the opportunity to use items of PPE to protect them from infectious agents.
A decline by any visitor should be respected, following an explanation of potential risks, and the visit allowed to maintain the resident’s ability to spend time with those important to them.
Advice on the correct use of PPE should be provided, but its use by visitors cannot be enforced.
Table 1: PPE use for care home visitors
| PPE Item to offer | Gloves | Aprons | Face mask | Goggles, visors, face shields for eye/face protection |
|---|---|---|---|---|
| When to use PPE | If providing direct care which exposes them to blood and/or body fluids for example assisted toileting or feeding. | To protect clothing if they intend to provide direct care which will expose them to someone’s blood/ or bodily fluids, or there is likely to be splashing of blood or bodily fluids during care delivery | To protect their nose and mouth from likely splash/spray of someone’s blood or bodily fluids or if they are entering the room of a resident with any suspected or known respiratory infection | To protect their face and eyes from any likely splash or spray from someone’s blood or bodily fluids |
 Read the PPE literature reviews to find out more information about the evidence base for PPE use.
Read the PPE literature reviews to find out more information about the evidence base for PPE use.
5. Safe management of care equipment
Care equipment is easily contaminated with blood, other body fluids, secretions, excretions and infectious agents and this can spread infection.
Important words and what they meanRoutine cleaning is regular cleaning which is carried out on a scheduled basis, not on an unplanned basis and not in response to an outbreak. For routine cleaning general purpose detergent and water solution or detergent impregnated wipes are sufficient.Cleaning is the removal of any dirt by use of an appropriate cleaning agent such as detergent.Decontamination is removing, or killing pathogens on an item or surface to make it safe for handling, re-use or disposal, by cleaning, disinfection and/or sterilisation.Disinfectant is a chemical used to reduce the number of infectious agents from an object or surface to a level that means they are not harmful to health.Detergent is a chemical cleansing agent that can dissolve oils and remove dirt.
If the resident has a known infection or the equipment is contaminated with blood or body fluids, then a disinfection agent needs to be used.
Note:
Do not use household bleach as the required dilution cannot be guaranteed.
Do not refill bottles for cleaning products as there is a risk of contamination.
What you need for safe management of care equipment
- Cleaning/disinfectant products:
- general purpose detergent and water solution/detergent impregnated wipes
or
-
- combined detergent/disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl.)
or
-
- a general purpose neutral detergent in a solution of warm water followed by disinfection solution of 1,000ppm av.cl.
- Paper towels/disposable cloths.
Types of equipment
There are three different types of care equipment that you will use in your care home and it is important that you know how to deal with each type.
You should follow manufacturers guidance for all equipment and products you use including those used for cleaning and decontamination.
Before using any sterile equipment, you should check that:
- the packaging is intact
- there are no obvious signs of packaging contamination
- the expiry date remains valid
1. Single-use - equipment which is used once on a single resident and then discarded.
Single-use equipment must never be reused even on the same resident. The packaging carries the symbol.

Note:
 Needles and syringes are single-use devices. They should never be used for more than one resident or reused to draw up additional medication.
Needles and syringes are single-use devices. They should never be used for more than one resident or reused to draw up additional medication.
Never give medications from a single-dose vial or intravenous (IV) bag to multiple residents.
2. Single individual use – equipment which can be reused by same resident for example a sling and decontaminated following use as per manufacturers instructions.
3. Reusable non-invasive equipment (often referred to as ‘communal equipment’) – equipment which can be reused on more than one resident following decontamination between each use for example commode, moving and handling equipment or bath hoist.
Cleaning or decontaminating reusable non-invasive equipment
Residents should be given their own reusable (communal) non-invasive equipment where possible.
Reusable equipment should be checked frequently for cleanliness and signs of integrity. This will include mattresses and pillows which should be clean, have a waterproof covering which is in a good state of repair.
Pillows used on resident’s beds may not require a waterproof cover if they are single resident use and are subject to regular checks/laundering. Resident pillows may require labelling where appropriate.
Reusable equipment should be cleaned or decontaminated:
- between individual use
- after blood and/or body fluid contamination
- as part of the regular scheduled cleaning schedules/process
- before inspection, servicing or repair
Staff should:
- follow the local cleaning protocol/schedule which should include responsibility for, frequency of and method of decontamination required
- use a general purpose detergent and water solution/detergent impregnated wipes
or
a combined detergent/disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl.)
or
a general purpose neutral detergent in a solution of warm water followed by disinfection solution of 1,000ppm av.cl;
- make up cleaning/disinfection solution following manufacturers guidance
- follow the manufacturer’s contact time for the cleaning/disinfection solution
- rinse and dry reusable equipment then store it clean and dry
Note: When an organisation use products or adopts practices that differ from those stated in this manual, that individual organisation is responsible for ensuring safe systems of work including the completion of risk assessments approved through local governance procedures.
Resources
 Read the management of care equipment literature review to find out more about why we do things this way for care equipment.
Read the management of care equipment literature review to find out more about why we do things this way for care equipment.
 The decontamination of non-invasive care equipment poster can help staff decide how to clean equipment.
The decontamination of non-invasive care equipment poster can help staff decide how to clean equipment.
Select image for full size version
6 - Safe management of the care environment
 There are many areas in care homes that become easily contaminated with microorganisms (germs) for example door handles, toilets, waste bins, surfaces.
There are many areas in care homes that become easily contaminated with microorganisms (germs) for example door handles, toilets, waste bins, surfaces.
Furniture and floorings in a poor state of repair can have microorganisms (germs) in hidden cracks or crevices.
To reduce the spread of infection, the environment should be kept clean and dry and where possible clear from clutter and equipment.
Non-essential items should be stored and displayed in such a way as to aid effective cleaning
Keeping a high standard of environmental cleanliness is important in the care home as the residents are often elderly and vulnerable to infections.
The care home environment should be:
- visibly clean, free from non-essential items and equipment to help make cleaning effective
- well maintained and in a good state of repair
- routinely cleaned in accordance with the specified cleaning schedules:
- a fresh solution of general purpose neutral detergent in warm water is recommended for routine cleaning. This should be changed when dirty or at 15 minutes’ intervals or when changing tasks
- routine disinfection of the environment is not recommended. However, 1,000 parts per million available chlorine (ppm available chlorine (av.cl.) should be used routinely on sanitary fittings
Staff should:
- report any issues with the environment cleanliness or maintenance to the person in charge to ensure that the care environment is safe. The person in charge should then act on problems reported to them
- be aware of the environmental cleaning schedules and clear on their specific responsibilities
Cleaning schedules should include:
- staff responsibilities
- cleaning frequencies
- cleaning methods
Managing cleaning services
Cleaning services should be managed in a systematic way, and staff responsible for cleaning should be appropriately trained to carry out the tasks they are responsible for.
The care home manager is responsible for managing the cleaning service which has a number of essential elements outlined in the cleaning services diagram.
Select the Care Homes Cleaning Specification for full size version of cleaning services diagram.
Select the diagram for full size version
Cleaning Services
An effective service will include all of the elements above.
Care Homes Cleaning Specification
The Care Homes Cleaning Specification provides a guide to planning cleaning services. It has tools to help with the planning and recording of cleaning activities and with the management activities marked with a * in the diagram above. These include:
- a structure to identify all spaces within a care home and plan appropriate cleaning tasks and frequencies
- a set of weekly and monthly cleaning templates to be assigned to each space within a care home. These can be used to develop a schedule and to provide a method for recording all cleaning activity
The tools within the Cleaning Specification should be used by the care home manager in the planning, training of staff, delivery, and checking of standards of the cleaning services they provide.
Manufacturer’s instructions and recommended contact times should be adhered to.
Cleaning schedule and record
Table 2 provides an example of a cleaning schedule and record. These tools are examples and designed to support local practice, however care homes can use their own tools if preferred. If a local tool is used, it should reflect the standards set out in the Care Homes Cleaning Specification.
Table 2: Example cleaning schedule residents room
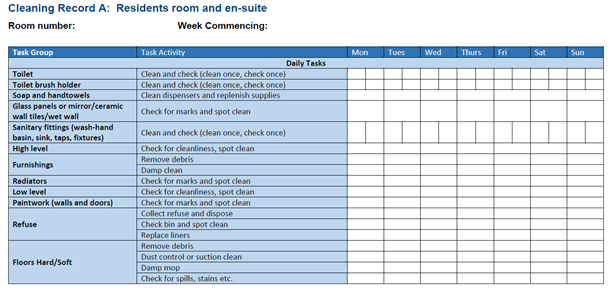
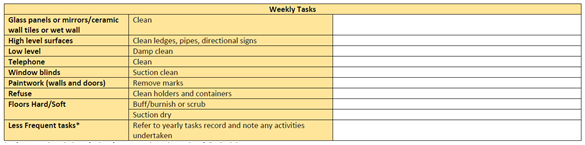
Standard operating procedures (SOPs) for all cleaning tasks.
Each SOP outlines the correct equipment, safety considerations, method, and outcomes required for each task. Table 3 shows the important steps that must be taken during the cleaning of floors.
Table 3: Example cleaning SOP: Floors
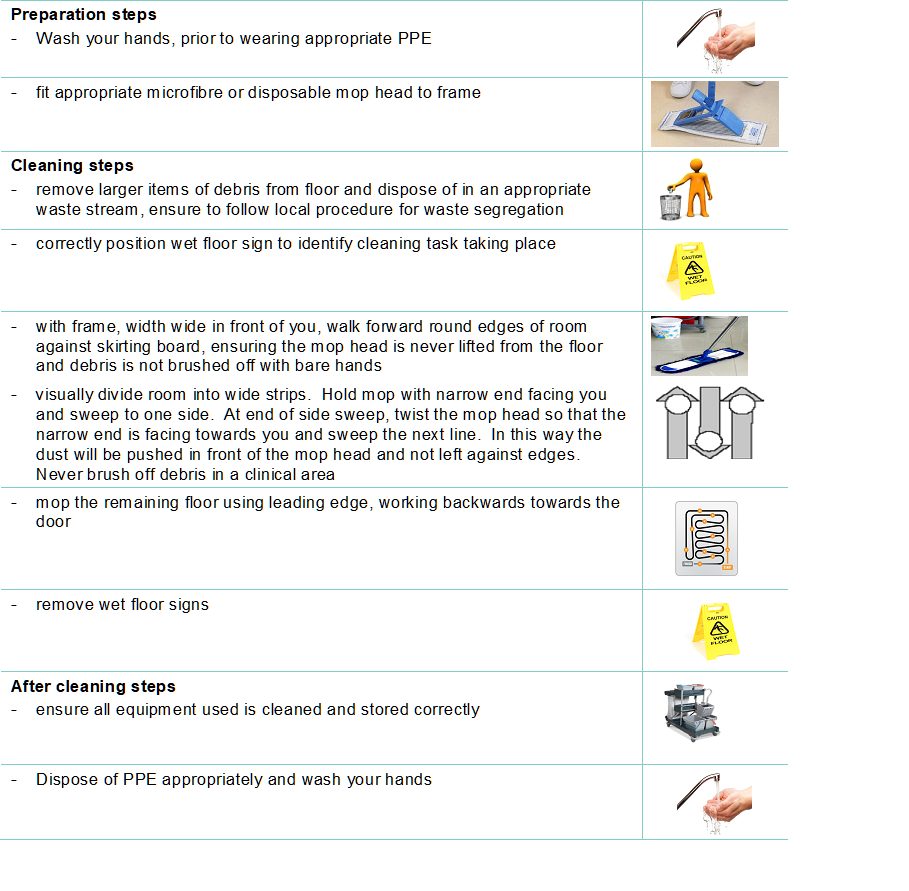
Process for checking
A process for checking the cleanliness of the care environment, to ensure standards are being maintained and to identify areas for improvement.
Decontamination of soft furnishings
Decontamination of soft furnishings may require to be discussed with the local HPT/ICT. If the soft furnishing is heavily contaminated with blood or body fluids, it may have to be discarded. If it is safe to clean with standard detergent and disinfectant alone then follow appropriate procedure.
If the item cannot withstand chlorine releasing agents staff are advised to consult the manufacturer’s instructions for a suitable alternative to use following or combined with detergent cleaning.
Note: When an organisation adopts decontamination processes not recommended in the CH IPCM the care organisation is responsible for governance of and completion of local risk assessment(s) to ensure safe systems of work.
 Read the routine cleaning of the care environment literature review to find out more about why we do things this way for the care environment.
Read the routine cleaning of the care environment literature review to find out more about why we do things this way for the care environment.
7 - Safe management of linen
Examples of linen you may have in the care home includes:
- bed linen (bed sheets, duvet, duvet covers, pillowcases)
- blankets
- curtains
- hoist slings
- towels
- resident clothing
There are three categories of linen:
Clean – Linen washed and ready for use
Used – All used linen in the care setting not contaminated by blood or body fluids
Infectious – All linen used by a person known or suspected to be infectious and/or linen that is contaminated with blood or body fluids for example faeces
Used or infectious linen may also be categorised as heat-labile: usually personal clothing where the clothing may be damaged (shrinking/stretching) by washing at a higher than recommended temperature than the label advises and therefore, cannot be subject to thermal disinfection. If such linen needs to be washed at a higher temperature for example if soiled or resident has a known infection they or their relatives need to be advised that the clothing may be damaged.
All clean, used and infectious linen should be handled with care and attention paid to the potential spread of infection. Appropriate temperatures for processing all used and infectious linen should be adhered to achieve thermal disinfection.
Clean linen
- Should be stored in a clean, allocated area. This should be an enclosed cupboard but a trolley could be used as long as it is completely covered with a waterproof covering that is able to withstand cleaning.
- Perform hand hygiene, in accordance with Section 2, prior to handling clean linen.
Used linen
Staff should:
- refer to Appendix 15 for decision making regarding PPE use
- check that linen is free from inappropriate items before placing into the laundry receptacle, for example used equipment, service user personal belongings
- make sure that a laundry trolley or container is available as close as possible to the point of use for immediate linen deposit
Staff should not:
- rinse, shake or sort linen on removal from beds or trolleys
- place used linen on the floor or any other surfaces for example on a locker or tabletop
- re-handle used linen once bagged
- overfill laundry receptacles or trolleys
Infectious linen
Staff should:
- wear disposable gloves and apron before handling infectious linen
- put infectious linen directly into a water soluble laundry bag and secure before putting into a clear plastic bag and placing into a laundry receptacle/trolley
If using external laundry services both used and infectious linen bags/receptacles should follow local procedure and arrangements. Store all used/infectious linen in a designated, safe, lockable area whilst awaiting uplift.
All linen that is deemed unfit for re-use, for example torn or heavily contaminated, should be categorised at the point of use and disposed of in the appropriate local healthcare waste stream.
Washing residents personal linen
Appendix 1 National Guidance for Safe Management of Linen in NHSScotland Health and Care Environments - For laundry services/distribution contains information that is particularly relevant and may be useful for residential care settings where domestic-type (household) washing machines may be in place for laundering resident’s personal items and clothing.
Domestic-type washing machines are not typically programmed with the temperature settings required for thermal disinfection, therefore domestic-type machines may only be used for laundering personal items of clothing belonging to residents, such as those that are heat-labile.
Other types of used linen such as sheets should be reprocessed using a machine that is capable of a validated temperature disinfection stage.
If using a domestic type washing machine to launder resident’s personal items:
- wash items using the highest temperature you can and following the washing instructions
- use your normal washing powder or detergent and follow the instructions on the correct amount to use
- tumble-dry (if possible) following the washing instructions
- iron according to washing instructions. If possible, use a hot steam iron.
It is considered best practise to launder a resident’s personal items separately, that means not to mix items from multiple persons within a single load.
If visitors wish to take their relatives clothes home to be laundered, place laundry in an appropriate bag and provide them with a washing clothes at home leaflet.
If the residents clothing is very soiled or infectious, staff may recommend that the clothing is washed in the care home’s laundry service if available, otherwise, the item should be disposed of in the appropriate healthcare waste stream following discussion with the resident or their relative(s).
 Read the safe management of linen literature review to find out more about why we do things this way when dealing with linen.
Read the safe management of linen literature review to find out more about why we do things this way when dealing with linen.
8 - Blood and body fluid spillages
Spillages of blood and other body fluids may transmit blood borne viruses.
Important words and what they meanA blood borne virus is a virus carried or transmitted by blood, for example Hepatitis B, Hepatitis C and HIV.Body fluids are fluids produced by the body such as urine, faeces, vomit or diarrhoea. These body fluids may also contain blood.
Blood and body fluid spillages must be decontaminated:
- immediately by staff trained to undertake this safely
- using body fluid spill kits/equipment available
Responsibilities for the decontamination of blood and body fluid spillages should be clear within each area/care setting.
Resources
 Read the management of blood and body fluid spillages literature review to find out more about why we do things this way for blood and body fluid spillages.
Read the management of blood and body fluid spillages literature review to find out more about why we do things this way for blood and body fluid spillages.
 Use the poster management of blood and body fluids to help you when you clean up blood and body fluid spillages.
Use the poster management of blood and body fluids to help you when you clean up blood and body fluid spillages.
Select the image for full size
9 - Safe disposal of waste (including sharps)
Classification of waste
Waste regulations require the classification of waste based on hazardous characteristics.
- Special (hazardous) waste. Special waste includes a range of controlled wastes, defined by legislation, which contain dangerous or hazardous substances. Examples of special (hazardous) waste resulting from healthcare activities includes sharps, infectious or potentially infectious clinical waste and some pharmaceuticals or medicinal wastes.
- Non-hazardous waste is residual waste produced in both clinical and non-clinical settings which may include dry recyclates (glass, paper and plastics, metals, cardboard), food waste, packaging waste and furniture.
Segregation (separating) of waste
Waste bags in care homes should be colour coded to denote the different waste streams.
Different types of waste will be produced within care homes.
Some waste may be considered non-hazardous, for example paper hand towels, while other types of waste need special handling and disposal because of their hazardous properties for example, sharps and waste from service users who have or may have an infection.
SHTN 03-01 contains a full colour-coded waste segregation guide however, the most frequently used waste streams are summarised below.
- Black (non-hazardous)
- residual waste remaining after all source segregated recyclates have been removed.
- Orange (infectious)
- consists of infectious or potentially infectious substances or items.
- orange lidded leak resistant receptacles may be used for solidified infectious liquids.
- orange bags may be used for items such as PPE, spillage kits, swabs or dressings contaminated or likely to be contaminated with blood and/or body fluids including saliva.
- orange lidded sharps box used for sharps disposal only
Local risk assessed processes for waste disposal should be followed and guidance from local contractors may apply.
Safe management of waste
Care home staff should ensure:
- all waste is managed according to relevant legislation and any local risk assessed processes
- the appropriate PPE is used when handling any special (hazardous) waste
- waste is disposed of as close to the point of use as possible, and segregated using the correct colour-coded waste bag or container compliant with UN and relevant industry standards
- liquid waste is disposed of via the toilet or macerator, or where this is not possible, solidified and placed in an appropriate rigid leak resistant receptacle
- clearly marked and secure containers for sharps disposal are available where sharps are used
- schedules are in place for the cleaning, emptying and uplifting of waste bins and waste does not accumulate in corridors, rooms, care areas or other publicly accessible areas
- waste bags are never overfilled and have been appropriately sealed, labelled and marked with the date and location before being stored for uplift.
- waste bags are securely sealed using a closure technique such as a ‘swan neck’
- a ‘swan neck’ is a way of closing bag by tying in a loop and securing with a zip tie or tape to make a handle
- there is a dedicated area for the storage of waste that is secure and not accessible to residents or the public
- any PPE is removed, and hand hygiene is performed after handling waste
 Read the safe disposal of waste literature review to find out more about why we do things this way when dealing with waste.
Read the safe disposal of waste literature review to find out more about why we do things this way when dealing with waste.
10. Occupational Safety: Prevention and Exposure Management (including sharps)
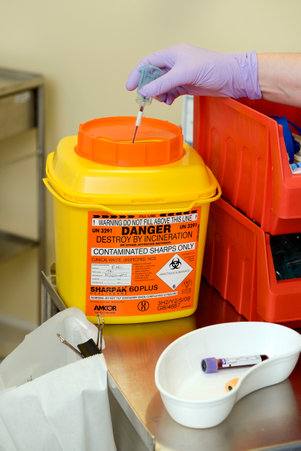 All care homes should have policies in place to ensure that staff are protected from occupational exposure to microorganisms (germs), particularly those that may be found in blood and body fluids.
All care homes should have policies in place to ensure that staff are protected from occupational exposure to microorganisms (germs), particularly those that may be found in blood and body fluids.
Important words and what they meanOccupational exposure is exposure of staff to blood or body fluids in the course of their work.A sharp is a device or instrument such as needles, lancets and scalpels which are necessary for the exercise of specific healthcare activities and are able to cut, prick and/or have the potential to cause injury.Safety device or safer sharp is a medical sharps device which has been designed to incorporate a feature or mechanism that minimises and/or prevents the risk of accidental injury. Other terms include (but are not limited to) safety devices, safety-engineered devices and safer needle devices.
The Health and Safety (Sharp Instruments in Healthcare) Regulations (2013) outline the regulatory requirements for employers and contractors in the healthcare sector in relation to:
- arrangements for the safe use and disposal of sharps
- provision of information and training to employees
- investigations and actions required in response to work related sharps injuries
Safe management of sharps in the care home
Sharps handling must be assessed, kept to a minimum and eliminated if possible with the use of approved safety devices.
Sharps safety
- Always dispose of needles and syringes as a single unit immediately at the point of use.
- Always assemble and label sharps containers correctly as per manufacturers instructions.
- Always use the temporary closure mechanisms on sharps containers in between use.
- Always follow manufacturers’ instructions for safe use and disposal.
- Never re-sheath used needles or lancets.
- Never store sharps containers on the floor.
- Never allow access of sharps containers to residents or the public.
- Never fill sharps containers more than three-quarters full.
Significant occupational exposure
A significant occupational exposure is when someone is injured at work from using sharps or exposed to risk from blood or body fluids which may then result in a blood borne virus (BBV) or other infection.
Examples of this would be:
- a percutaneous injury for example injuries from needles, instruments, bone fragments, or bites which break the skin
- exposure of broken skin for example abrasions, cuts, eczema
- exposure of mucous membranes including the eye from splashing and/or spraying of blood or bodily fluids
If you think or know you have had a significant occupational exposure you should:
- report this immediately to the designated person in your care home, this is a legal requirement
- follow the local agreed process for management of an occupational exposure incident and follow the management of occupational injuries flow chart
Resources
 Read the management of occupational exposure to Blood Borne Viruses (BBVs) literature review to find out more about why we do things this way for occupational exposure.
Read the management of occupational exposure to Blood Borne Viruses (BBVs) literature review to find out more about why we do things this way for occupational exposure.

The management of occupational exposure incidents flowchart should be used within your care home so you know what to do for an occupational exposure.
Select the image for full size
Transmission-based precautions (TBPs) definitions literature review update
Why are transmission descriptors (contact, droplet, airborne) being reviewed?
The pandemic highlighted that the way in which respiratory transmission is currently described (droplet and airborne transmission) may not reflect what is happening in real life and so, there is a need to identify alternatives ways to describe respiratory transmission routes.
Understanding how infectious agents are released into the air and the risks associated with particle size and distance from source will help inform this. Reviewing the evidence to understand if there is increased risk associated with certain medical procedures will also inform IPC practice.
The World Health Organization (WHO) and Centers for Disease Control (CDC) have also reviewed transmission descriptors indicating a global shift in the way transmission routes are described. ARHAI Scotland were invited to meet with the WHO global IPC unit to discuss the topic and our literature review findings were well received.
What is likely to change in the CHIPCM?
ARHAI are currently developing recommendations for practice. It is likely that ‘droplet transmission’ and ‘airborne transmission’ will be replaced with new definitions to describe respiratory transmission. This will mean changes throughout the CHIPCM to update the terminology including the addition of resources to support any guidance changes.
What might this mean for care home staff in practice?
It is too early to understand what might change in practice, but it is likely that there will be a need for care home staff to consider more factors when risk assessing what PPE to wear.
The goal of the CHIPCM is to provide care home staff in Scotland with guidance that is evidence based, up-to-date, effective, practical, and as a result, safe.
Update announcements
Use of the CHIPCM online is always advised to ensure access to up to date guidance. Updates to the CHIPCM content are communicated to stakeholders via the Care Home IPC Oversight and Advisory Group in addition to the news section of the NIPCM.
For further detail please use this link to the Transmission Based Precautions Definitions Literature Review.
Transmission based precautions (TBPs)
In certain circumstances using standard infection control precautions (SICPs) won’t be enough to stop an infection spreading and you will need to use some extra precautions. These extra precautions are called Transmission Based Precautions or TBPs.
Clinical judgement and decisions should be made by staff to determine the necessary IPC precautions required (the local IPCT and/or the HPT should be contacted for advice and support where required).
Clinical judgement and decisions should be based on the:
- suspected or known infectious agent
- transmission route of the infectious agent
- care setting and procedures undertaken
- severity of the illness caused
When TBPs should be used
TBPs should be used if a resident has a suspected or known infection or colonisation.
Important words and what they meanColonisation is the presence of microorganisms on a body surface (such as the skin, mouth, intestines or airway) that does not cause disease in the person or signs of infection.
Infection transmission routes
Infections can be transmitted or spread by:
- contact with microorganisms (germs) on hands or from contaminated equipment or environment
- droplet infection by inhaling infectious droplets, for example flu or COVID-19
- aerosol infection which are inhaled or directly contaminate a mucosal surface or conjunctivae (eyes, nose, and mouth)
Different transmission routes need different TBPs
TBPs are categorised by the route of transmission of infectious agents (some infectious agents can be transmitted by more than one route). Appendix 11 provides details of the type of precautions, optimal resident placement, isolation requirements and any respiratory precautions required. Application of TBPs may differ depending on the setting and the known or suspected infectious agent.
- Contact precautions are used to prevent infections that spread through direct contact with the resident or indirectly from the resident’s immediate care environment and care equipment. This is the most common route of cross-infection transmission.
- Droplet precautions are used to prevent and control infections spread over short distances (at least 3 feet or 1 metre) via small droplets from the respiratory tract of one individual directly onto the mucosal surface of another person’s mouth or nose or eyes. Droplets penetrate the respiratory system to above the alveolar level.
- Airborne precautions are used to prevent and control infections spread without necessarily having close contact via from the respiratory tract of one individual directly onto the surface of another person’s mouth or nose or eyes. Aerosols penetrate the respiratory system to deep into the lung.
Different infections need different TBPs
You might have heard of some infections like norovirus, Meticillin-resistant Staphylococcus aureus (MRSA), Clostridioides. difficile (C.diff/CDI) and flu but there are lots of others.
You can find out more information about the infection the individual has and the precautions you should use in Appendix 11 and/or A-Z of pathogens in the NIPCM.
You can also contact your local IPCT or HPT for further advice if required.
Before using transmission based precautions you need to find out:
- what the suspected or known infection/colonisation is?
- how is it transmitted?
- how severe is the resident’s illness?
- what is the care setting and required procedures?
There are different ways you can find out if a resident has an infection that needs TBPs to be put in place. You can get information about a resident’s infection status from:
- their GP
- local health protection team
- local infection prevention and control team
- laboratory
- hospital or care homes staff from where the resident has been discharged or transferred
Local processes should be followed when obtaining this information.
![]() Further information on transmission based precautions can be found in the definitions of Transmission Based Precautions literature review.
Further information on transmission based precautions can be found in the definitions of Transmission Based Precautions literature review.
1. Resident placement/assessment for infection risk
All residents require to be regularly monitored for infection throughout their stay for the correct IPC precautions to be put in place to minimise the risk of infection being spread.
Residents may be an infection risk if they have:
- diarrhoea, vomiting, an unexplained rash, fever or respiratory symptoms
- a known (laboratory confirmed) or suspected infectious pathogen for which appropriate duration of precautions as outlined in A-Z of pathogens are not yet complete
- been previously positive with a Multi-drug Resistant Organism (MDRO) for example Meticillin-resistant Staphylococcus aureus (MRSA); Carbapenemase Producing Enterobacterales (CPE)*
*CPE should be considered if the resident meets any of the following criteria within the 12-month period before admission:
- been an inpatient in a hospital outside of Scotland
- received holiday dialysis outside of Scotland
- been a close contact of a person who has been colonised or infected with CPE.
See the CPE toolkit for non-acute settings for further information and requirements.
Staff should do the following if any resident displays signs and/or symptoms of infection:
- obtain advice on the resident’s clinical management from their GP and advice on appropriate IPC management from either the local HPT or IPCT
- make resident placement decisions based on advice received or sound judgement by clinical staff who are involved in the resident’s management
- advise the ambulance service of the resident’s infectious condition if transport to hospital is required
Residents should not be moved within the care home if they have signs and symptoms of infection unless essential.
Resident isolation requirements within the care home
Residents may require to be isolated in their own room because of a known or suspected infection. During this time it is important that:
- residents remain in their rooms whilst considered infectious and the door should remain closed. If it is not possible for safety reasons, for example the resident has dementia, an individual risk assessment should be done and any decisions or actions should be documented. The local HPT or IPCT should be contacted for advice
- suitable discrete signage is placed on the door advising others not to enter the room
- there should be as much consistency in staff allocation as possible to care for residents in isolation room areas as an additional IPC measure. This is known as ‘staff cohorting’
- resident isolation requirements should remain under continuous review considering individual risk factors and the impact on the resident. The local HPT or IPCT should be contacted for advice in these circumstances
Note: If a resident requires isolation because of infection or in an outbreak situation, this should be individually risk assessed to ensure the safety and health and wellbeing needs of the resident. Isolation periods must be monitored on daily basis and be for the minimum period specified.
 Read the patient placement, isolation and cohorting literature review to find out more about why we do things this way for resident placement for TBPs.
Read the patient placement, isolation and cohorting literature review to find out more about why we do things this way for resident placement for TBPs.
2. Safe management of care equipment in an isolation room
Cleaning and decontamination of care equipment is essential to reduce the spread of infection when infection is confirmed/suspected.
When dealing with the equipment used in the resident’s isolation room staff should:
- use single use or dedicated reusable care equipment, for example commodes, for the resident in isolation where possible
- clean and decontaminate all care equipment after each use as per Appendix 7
 Read the management of care equipment literature review to find out more about why we do things this way for patient care equipment for TBPs.
Read the management of care equipment literature review to find out more about why we do things this way for patient care equipment for TBPs.
3 - Safe management of the care environment
Isolation room cleaning
Staff should:
- clean and decontaminate the isolation room at least daily or more frequently if advised to do so. If you have been advised to clean more than daily this should be documented in the environmental cleaning schedule
- clean frequently touched surfaces like door handles, bed frames and bedside cabinets at least twice daily
- use correct cleaning products which are either:
a combined detergent/disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl.));
or
a general purpose neutral detergent in a solution of warm water followed by disinfection solution of 1,000ppm av.cl.
- follow manufacturers guidance and instructions on how to use the product and what the recommended contact time is for the product to work. This may include rinsing off the disinfection solution to prevent damage to surfaces.
Do not refill spray containers for cleaning products as there is a risk of contamination.
Terminal clean
A terminal clean is carried out when the resident is no longer considered infectious and/or when the environment is cleaned/decontaminated to ensure it is safe for a new resident.
A terminal clean is carried out by:
- removing all healthcare waste and other disposable items from the room
- removing bedding, curtains (bagged before removal from the room) and then wash as infectious linen
- cleaning and decontaminating all reusable care equipment in the room (prior to removal from the room)
The room should then be decontaminated using either:
- a combined detergent disinfectant solution at a dilution (1,000ppm av.cl.) or
- a general purpose neutral detergent clean in a solution of warm water followed by disinfection solution of 1,000ppm av.cl.
The room should be cleaned from the highest to lowest point and from the least to most contaminated point.
Manufacturers’ guidance and recommended product "contact time" should be followed for all cleaning/disinfection solutions.
 Appendix 7 is a poster flowchart for decontamination of reusable non-invasive care equipment that you may wish to print off and place in the care home.
Appendix 7 is a poster flowchart for decontamination of reusable non-invasive care equipment that you may wish to print off and place in the care home.
Note: When an organisation use products or adopts practices that differ from those stated in this manual, that individual organisation is responsible for ensuring safe systems of work including the completion of risk assessments approved through local governance procedures.
4. Personal Protective Equipment (PPE): Respiratory Protective Equipment (RPE)
In addition to PPE used for Standard Infection Control Precautions, appendix 15 of the NIPCM outlines what type of PPE and RPE you will need to wear for infections spread by different transmission routes.
Gloves
Gloves are a single-use item and should be donned immediately prior to exposure risk and should be doffed immediately after each use or upon completion of a task.
Gloves should:
- be worn when exposure to blood, body fluids, (including but not limited to secretions and/or excretions), non-intact skin, lesions and/or vesicles, mucous membranes, hazardous drugs, and chemicals for example cleaning agents, is anticipated/likely
- never be worn inappropriately in situations such as to go between residents, move around a care area, work at IT workstations
- be changed if a perforation or puncture is suspected or identified
- be appropriate for use, fit for purpose and well-fitting
- not be worn as a substitute to hand hygiene
- never be decontaminated with ABHR
Resources
 For appropriate glove use and selection see the flowchart poster which may be printed off and placed in the care home.
For appropriate glove use and selection see the flowchart poster which may be printed off and placed in the care home.
![]() Further information can be found in the Gloves literature review.
Further information can be found in the Gloves literature review.
Aprons/gowns
An apron should be worn when caring for residents known or suspected to be colonised/infected with antibiotic resistant bacteria including contact with the resident’s environment.
Plastic aprons should be used in care home settings for protection against contamination with blood and/or body fluids.
A fluid repellent gown should be used if excessive splashing or spraying is anticipated.
A full body fluid repellent gown should be worn when conducting AGPs on residents known or suspected to be infected with a respiratory infectious agent.
![]() Further information can be found in the Aprons/Gowns literature review.
Further information can be found in the Aprons/Gowns literature review.
Eye/face protection
Eye and face protection should be used in combination with:
- a fluid resistant Type IIR surgical mask when caring for symptomatic residents infected with droplet transmitted infectious agents
- a fluid resistant FFP3 respirator when caring for symptomatic residents infected with an airborne transmitted infectious agent
Eye/face protection should be worn:
- by all of those in the room if potentially infectious AGPs are conducted
- for the care of residents with novel infectious agents including pandemic influenza
 Read Appendix 11 for details of the type of precautions, optimal resident placement, isolation requirements and any respiratory precautions required.
Read Appendix 11 for details of the type of precautions, optimal resident placement, isolation requirements and any respiratory precautions required.
Surgical masks
A Type IIR fluid resistant surgical mask (FRSM) should be worn when caring for a resident with a suspected/confirmed infectious agent spread by the droplet route.
FRSMs should be worn (where tolerated) by residents with suspected/confirmed infectious agents spread by the droplet or airborne routes, as a form of source control and should meet type II or IIR standards.
 Read Appendix 11 for details of the type of precautions, optimal resident placement, isolation requirements and any respiratory precautions required.
Read Appendix 11 for details of the type of precautions, optimal resident placement, isolation requirements and any respiratory precautions required.
Respiratory Protective Equipment (RPE)
PPE should still be used in accordance with SICPs when using respiratory protective equipment (RPE). See Chapter 1.4 for PPE use for SICPs.
The use of FFP3s is governed by health and safety regulations and they must be fit tested to the user to ensure the required protection is provided.
The Health and Safety Executive (HSE) provides information regarding fitting and fit checking of RPE.
Respiratory Protective Equipment (RPE), for instance FFP3 and eye/face protection, should be considered when:
- a resident is admitted or develops a known/suspected infectious agent/disease spread wholly by the airborne route
- carrying out AGPs on residents with known/suspected infectious agent spread wholly or partly by the airborne or droplet route
FFP3 respirators
It is an HSE requirement that staff who need to wear an FFP3 respirator must be fit tested.
FFP3 respirators should not be worn by staff who are not trained in their use or who have not been fit tested.
All FFP3 respirators must be:
- fit tested (by a competent fit test operator) on all staff who may be required to wear a respirator to ensure an adequate seal/fit according to the manufacturers’ guidance
- fit checked (according to the manufacturers’ guidance) every time a respirator is donned to ensure an adequate seal has been achieved
- single-use (disposable) and fluid resistant
- compatible with other items of PPE and worn in accordance with manufacturer’s instructions. Prescription eyeglasses and contact lenses should not be considered a form of eye/face protection
- put on before entry into the resident’s room and removed in a safe area once outside the resident’s room
- changed after each use
Signs that a change in respirator is required include:
- breathing becomes difficult
- the respirator is wet or moist
- the respirator is damaged
- the respirator is obviously contaminated with body fluids such as respiratory secretions
Resources
 A poster containing information on compatibility of facial hair and FFP3 respirators can be used when fit testing and fit checking.
A poster containing information on compatibility of facial hair and FFP3 respirators can be used when fit testing and fit checking.
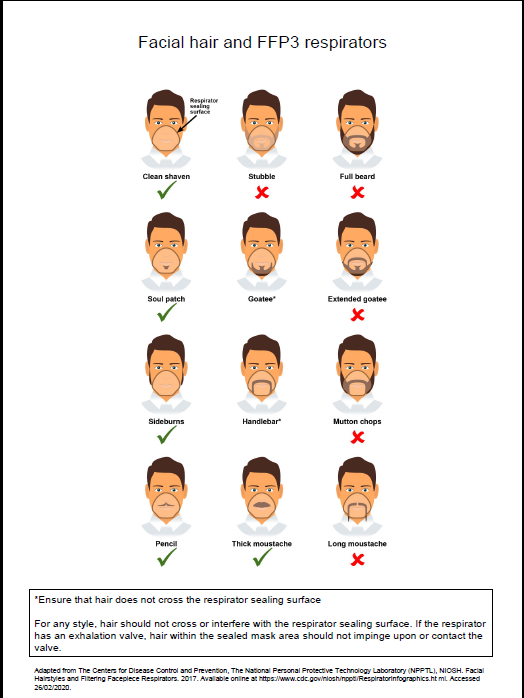
![]() Further information regarding fitting and fit checking of respirators can be found from the Health and Safety Executive
Further information regarding fitting and fit checking of respirators can be found from the Health and Safety Executive
Aerosol Generating Procedure (AGP)
An AGP is a medical procedure that can result in the release of airborne particles from the respiratory tract and is associated with an increased risk of transmission when treating someone who is suspected or known to be suffering from an infectious agent transmitted wholly or partly by the airborne or droplet route.
The most common AGPs undertaken in the Care Home Setting are Continuous Positive Airway Pressure Ventilation (CPAP) or Bi-level Positive Airway Pressure Ventilation (BiPAP) or tracheostomy procedures (insertion or removal) and open suctioning beyond the oro-pharynx.
The full list of medical procedures that have reported to be aerosol generating and are associated with an increased risk of respiratory transmission can be found in appendix 16.
A poster is also available on PPE when undertaking AGPs within health and social care settings.
Rooms should always be decontaminated following an AGP. Clearance of infectious particles after an AGP is dependent on the ventilation and air change within the room. In an isolation room with mechanical ventilation 10-12 air changes per hour (ACH) a minimum of 20 minutes is required; in a side room with 6 ACH this would be approximately one hour.
It is often difficult to calculate air changes in areas that have natural ventilation only, meaning no mechanical ventilation. Natural ventilation, particularly when reliant on open windows can vary depending on the climate. An air change rate in these circumstances has been agreed as 1-2 air changes/hour.
Rooms should always be decontaminated following the completion of an AGP. Regardless of the number of air changes, a period of 10 minutes should pass to allow larger droplets to settle before the room can be cleaned. Staff are required to wear the appropriate PPE until the fallow time has been met.
Resources
![]()
For further information on fallow times refer to Table 1 in Appendix 16.
 Further information can be found in the literature reviews aerosol generating procedures, Respiratory Protective Equipment (RPE), Personal Protective Equipment (PPE) for High Consequence Infectious Diseases (HCID)
Further information can be found in the literature reviews aerosol generating procedures, Respiratory Protective Equipment (RPE), Personal Protective Equipment (PPE) for High Consequence Infectious Diseases (HCID)
Post AGP fallow time (PAGPFT)
Time is required after an AGP is performed to allow the aerosols still circulating to be removed/diluted. This is referred to as post AGP fallow time (PAGPFT) and is a function of the room ventilation air change rate.
The post aerosol generating procedure fallow time (PAGPFT) calculations are detailed in appendix 16. It is often difficult to calculate air changes in areas that have natural ventilation only.
If the area has zero air changes and no natural ventilation, then AGPs should not be undertaken in this area.
The duration of AGP is also required to calculate the PAGPFT and clinical staff are therefore reminded to note the start time of an AGP. It is presumed that the longer the AGP, the more aerosols are produced and therefore require a longer dilution time. During the PAGPFT staff should not enter this room without FFP3 masks. Other residents, other than the resident on which the AGP was undertaken, should not enter the room until the PAGPFT has elapsed and the surrounding area has been cleaned appropriately. As a minimum, regardless of air changes per hour (ACH), a period of 10 minutes should pass before rooms can be cleaned. This is to allow for the large droplets to settle. Staff should not enter rooms in which AGPs have been performed without airborne precautions for a minimum of 10 minutes from completion of AGP. Airborne precautions may also be required for a further extended period of time based on the duration of the AGP and the number of air changes. Cleaning can be carried out after 10 minutes regardless of the extended time for airborne PPE.
Contact your local HPT/IPCT if further advice is required.
 Read the RPE literature review to find out more about why we do things this way for respiratory protective equipment.
Read the RPE literature review to find out more about why we do things this way for respiratory protective equipment.
Use of FFP3 respirators
- Where staff have concerns, they may choose to wear an FFP3 respirator rather than a fluid-resistant surgical mask (FRSM) when providing patient care, provided they are fit tested. This is a personal PPE risk assessment.
5. Infection prevention and control during care of the deceased
If a resident dies when in the care home SICPs and TBPs should still be applied. This is due to the ongoing risk of infectious transmission via contact although the risk is usually lower than for the living.
It is important that information on the infection status of the deceased is sought and communicated at each stage of handling and risk assessments performed.
Viewing, washing and/or dressing of the deceased see Appendix 12 - Application of infection prevention precautions in the deceased for guidance on the precautions required and what is permitted for certain types of infections.
Staff should advise relatives of the appropriate precautions to be taken when viewing and/or having physical contact with the deceased, including when this should be avoided.
 Read the infection prevention and control during care of the deceased literature review to find out more about why we do things this way when dealing with the deceased.
Read the infection prevention and control during care of the deceased literature review to find out more about why we do things this way when dealing with the deceased.
A-Z pathogens
The A-Z provides a description of pathogen, incubation period and infectivity along with transmission routes, notifiable status and alert organisms for diseases associated with the pathogen. It is intended as a useful tool to guide local clinical risk assessment and decision making.
The A-Z also contains links to external UK/International guidance documents (non-Scottish); ARHAI Scotland is not responsible for the content of these guidance documents.
Care home appendices
These appendices from the NIPCM can be used in care homes.
Appendix 5 - Glove use and selection
Appendix 6 - Putting on and removing PPE
Appendix 7 - Decontamination of reusable non-invasive care equipment
Appendix 8 - Management of linen at care level
Appendix 9 - Management of blood and body fluid spillages
Appendix 10 - Management of occupational exposure incidents
Appendix 12 - Application of infection control precautions in the deceased
Appendix 16 - Aerosol generating procedures (AGPs) and post AGP fallow times (PAGPFT)
Appendix 17 - Hierarchy of controls
Care home resources
The resources section can be used as supporting tools for the Care Home Infection Prevention and Control Manual (CH IPCM).
- Aerosol Generating Procedures
- Care Home Infection Prevention and Control Resource Toolkit, (v6.0, January 2026)
- Catheter Associated Urinary Tract Infection (CAUTI)
- HAI Compendium
- Hand hygiene
- Gastro-intestinal illness
- Information leaflets.
These leaflets are also available in different languages National Infection Prevention and Control Manual: Information Leaflets - language translations (scot.nhs.uk)- Clostridiodes difficile (CDI) - Information for people who are getting care, their visitors, and anyone else who is in a care setting
- Group A Streptoccocal infection - information for patients
- Healthcare associated infection (HAI) - Information for the public
- Vancomycin-resistant enterococci VRE - Information for patients
- Washing clothes at home - Information for people in hospitals or care homes and their relatives
- Norovirus - key steps to help stop the spread of infection poster
- Respiratory hygiene
- Scabies: Back to Basics Presentation - 23 October 2024
- Transmission Based Precautions
- Waste Management: Back-to-Basics webinar - 12 December 2024
- Winter Preparedness Campaign 2025
How to contact us
If you have any questions or feedback about the Care Home IPCM then you can contact us by email or telephone.
Telephone: 0141 300 1175
Evidence and research
Evidence Based Content
The recommendations for practice made in the NIPCM are informed by living systematic literature reviews.
Literature reviews are undertaken following defined processes for evidence identification and appraisal, and development of recommendations for practice which are then translated into NIPCM content. These processes follow a defined governance structure, details of these are found in the Development Methodology.
Please note that in the updated Development Methodology (March 2025), appendix 5 containing all search strategies has been removed. We are currently in the process of moving individual search strategies to their appropriate literature reviews.
The scientific evidence for the NIPCM is reviewed on a real time basis and details of the reviews reported quarterly.
Any changes identified in the scientific literature may lead to a change being made to the NIPCM following stakeholder engagement.
Evidence Documents
There are four evidence documents associated with each literature review:
Literature Reviews. Provide a comprehensive systematic review and description of the evidence.
Evidence tables. These detail all the included studies and provide an assessment of the evidence for each research question of the literature review.
Considered judgement forms. Outline the evidence base and expert opinion used to develop the recommendations and good practice points for each literature review research question. Also detailed are the benefits, potential harms, feasibility of implementation, value judgements, intentional vagueness, and exceptions associated with the recommendations and good practice points.
Executive Summary. These give an overview of the scope of the literature review, key highlights and notable changes to recommendations and good practice points. They highlight any major changes to NIPCM content expected as a result of a literature review update. A full list of the recommendations and good practice points are provided in the executive summary.
Literature reviews
- Decontamination and built environment
- Hand hygiene
- Incidents and outbreaks
- Management of care equipment
- Management of the care environment
- Patient management
- Pandemic response
- Personal Protective Equipment (PPE)
- Transmission Based Precautions definitions
NIPCM Quarterly Evidence Reviews
The scientific evidence for the NIPCM is reviewed on a real time basis and details of the reviews reported quarterly.
2025
2024
2023
2022
2021
SICPs and TBPs
2020
2019
SBAR tools
Statement: Please note that SBARs published before December 2023 make reference to alcohol based hand rub (ABHR). As of date 11 January 2024 this is now referred to as hand rub in the NIPCM based on the updated recommendations in the hand products literature review (with the exception of surgical hand antisepsis which has its own body of research).
An SBAR (Situation, Background, Assessment, Recommendation) is a communication tool presented in four standardised sections that allow organisations to present key information and communicate it in a clear and concise way. SBARs are often used in healthcare settings to:
- facilitate communication about patient care between healthcare workers, and/or
- present the results of an evidence review to inform evidence-based practice in healthcare settings.
View ARHAI SBARs
ARHAI Scotland Publications
ARHAI Scotland have published in scientific journals
- Cryptococcus neoformans/Cryptococcus gattii species complex infections with recommendations for practice in health and care settings, (2022)
- Infection prevention and control factors associated with post-cataract surgery endophthalmitis - a review of the literature from 2010 – 2023, (2024)
- Candidozyma auris Reported in Scotland: a Call for Vigilance Amid Global Rise, (2026)
COVID-19 NIPCM Archives
This page links to the following archived COVID-19 guidance that was in the NIPCM:
- Acute addendums
- Community addendums
- Care Home addendums
- Appendix 21 - COVID-19 Pandemic controls for Acute Settings including Scottish Ambulance Service
- Appendix 22 - Community Infection Prevention and Control COVID-19 Pandemic
- Appendix 21 - COVID-19 Pandemic controls for health and care settings
- Hospital Testing Tables
- Literature reviews and rapid reviews
Public Health Scotland hold the archive of COVID-19 Guidance and Publications.
COVID-19 Acute Addendum Archives
Version |
Date |
Summary of changes |
|
26/10/2020 |
First publication |
|
|
28/10/2020 |
Update to section 5.7 ‘Safe Management of the Care Environment’ to reflect detail of 2nd daily clean. Update to section 5.5 ‘Personal Protective Equipment’ to be more explicit. |
|
|
06/11/2020 |
Update to align references to changing of facemasks between pathways. |
|
|
20/11/2020 |
New section on communications when transferring a suspected/confirmed case New section on car sharing New section on visiting Update to definition of recovered patient |
|
|
09/12/2020 |
New section on PPE requirements for delivery of vaccinations New section on outbreaks |
|
|
17/12/2020 |
New section on COVID-19 testing New section on Patients returning from weekend/day pass New section on Whole Genome Sequencing (WGS) Link to RCPCH paediatric guidance for pre-operative admission assessment and testing requirements New FRSM poster (ways to improve fit) |
|
|
23/12/2020 |
Update to 5.0.3 to reflect changes in stepdown guidance Inclusion of SG link to asymptomatic staff testing information New section on 5.1.1 Non-COVID patient transfers between different wards and hospitals |
|
|
22/01/2021 |
Update to the COVID-19 testing section and associated testing table New section on guidance for the Discontinuation of Infection control precautions and discharging COVID-19 patients from hospital Update to PPE guidance specifically in relation to visors New section on the hierarchy of controls |
|
|
18/02/2021 |
Update to resources and Rapid reviews content Additional wording added to definition of suspected case section to reflect wide variety of presenting symptoms Strengthening of triage question relating to travel history Additional paragraph in PPE section reinforcing need for visiting staff to seek clarity on patient pathway and PPE requirements prior to patient contact |
|
|
26/03/2021 |
Sessional PPE use no longer accepted beyond eye protection in the high risk pathway and FRSMs across all pathways. Update to stepdown requirement for inpatient table to recognise need for clinical assessment Useful tools section |
|
|
07/05/2021 |
Environmental risk assessment |
|
|
14/05/2021 |
Change to AGP list to remove upper airway suctioning during Upper GI Endoscopy and replace with suctioning beyond the oro-pharynx. |
|
|
18/05/2021 |
Update to COVID-19 testing table to reflect the need to test all contacts of confirmed cases. |
|
|
25/06/2021 |
Update to PPE table to emphasise Risk Assessment in low and medium risk pathway Addition of risk associated with valved respirators Change in controls for management of linen, waste and environmental cleaning from TBPs to SICPs within the Medium Risk pathway |
|
|
8/7/2021 |
5.2 COVID-19 testing. Update made to include 'or the first positive test, if asymptomatic or other symptoms, unless they develop new possible COVID-19 symptoms' regarding any patient who has previously tested positive for SARS-CoV-2 by PCR. 5.3.8 Update to table 2 - stepdown table for 'Patient discharging to a care facility including nursing homes and residential homes' 5.3.8 Inclusion of section on 'Patients discharged from hospital to a care home (non-COVID-19) |
|
|
19/7/2021 |
Update to Hierarchy of control including risk assessment algorithm Inclusion of a specific paragraph advising on the use of FFP3 masks |
|
|
30/8/2021 |
Update to physical distancing |
|
|
|
29/11/2021 |
Replaced by Version 1.0 of the respiratory addendum |
Scottish COVID-19 Infection Prevention and Control Addendum for Community Health and Care Settings
Version |
Date |
Summary of changes |
|
07/01/2021 |
First version |
|
|
25/01/2021 |
Addition of section 7.2.5 'Discontinuing IPC control measures in community health and care settings for COVID-19 individuals' |
|
|
31/03/2021 |
Health Centres included in list |
|
|
08/07/2021 |
7.5.5 Change to AGP list to remove upper airway suctioning during Upper GI Endoscopy and replace with suctioning beyond the oro-pharynx. |
|
|
25/08/2021 |
Inclusion of dental services within the addendum |
|
|
31/08/2021 |
Update to physical distancing |
|
|
15/09/2021 |
Update to physical distancing to include further information for visitors and residents within residential homes. |
|
|
|
29/11/2021 |
Replaced by Version 1.0 of the respiratory addendum |
COVID-19 Care Home Addendum Archive
Version |
Date |
Summary of changes |
|
16/12/2020 |
First version |
|
|
25/01/2021 |
Inclusion of new section 6.2.4 'Discontinuing IPC precautions in care homes for residents who are COVID-19 positive' |
|
|
31/03/2021 |
6.1.2 Definition of suspected case; Additional information and links included. |
|
|
8 July 2021 |
6.1.3 Triaging of residents admitted to a care home updated with changes to testing and self-isolation |
|
|
31/08/2021 |
Updates to physical distancing |
|
|
|
29/11/2021 |
Replaced by Version 1.0 of the Respiratory Addendum |
Respiratory Addendum archive
Version |
Date |
Summary of changes |
|
29/11/2021 |
Guidance launched |
|
|
13/12/2021 |
Update to ‘Determining the IPC precautions required for AGPs’ |
|
|
17/01/2022 |
Addition of advice for regular testing in critical care units where AGPs are regularly performed on the non respiratory pathway. Reduction of COVID-19 duration of precautions from 14 days to 10 days. |
|
|
20/01/2022 |
Update to Non COVID-19 discharges (non respiratory pathway) from hospitals to care homes Addition of sections for primary care and care homes to reinforce and support assessment using the hierarchy of controls. |
|
|
03/02/2022 |
Additional information for visitors entering AGP zones. |
|
|
23/02/2022 |
Risk assessment for management of patient placement in long term residential community settings (section 5.8 and section 5.12.2) Update to hospital testing table |
|
|
01/04/2022 |
The following updates reflect changes to healthcare COVID-19 pandemic controls as outlined in DL (2022) 07 as follows; Changes to patient testing requirements including Hospital Testing Table Inclusion of the wider use of Rapid Diagnostic Testing (including POCT) or LFD testing Changes to management of contacts including inclusion of 28 day contact exemption Changes to respiratory screening questions Changes to testing requirement pre AGP on the non respiratory pathway Withdrawal of car sharing guidance Removal of physical distancing guidance Please note: the above changes within version 1.6 are not applicable in care homes, prisons and social community and residential care settings at the time of version update; Extant guidance remains in place for these settings. |
|
|
07/04/2022 |
Update to include definition of fully vaccinated Addition to physical distancing noting that services may choose to retain physical distancing where they deem it necessary |
|
|
27/04/2022 |
Addition of testing responsibilities at an organisational level and clarity of testing language Change to isolation advice for service users with COVID-19 Removal of vaccination as part of contact management. |
|
|
|
11/07/2022 |
The respiratory addendum was archived and relevant content included in the NIPCM |
Appendix 21 -COVID-19 Pandemic Controls for Acute NHS settings including Scottish Ambulance Service (SAS) Archive
Version |
Date |
Summary of changes |
|
10/05/2022 |
First publication – Marks transition from Winter Respiratory Infection IPC Addendum back to NIPCM. |
|
|
30/05/2022 |
Reference to COVID-19 screening removed. |
|
|
13/07/2022 |
Addition of dental services and GPs to title. |
|
|
28/07/2022 |
Removal of GPs in title as now included in Appendix 22. |
|
|
22/08/ 2022 |
Changes made to testing requirements in line with DL 2022(29) issued on 22nd August 2022 |
|
|
16/09/2022 |
Update following Directors Letter (2022)32 - pause in asymptomatic COVID-19 testing in health and social care |
|
|
28/09/2022 |
Removal of ‘Primary Care Settings’ from Table 2 – Respiratory Screening Questions |
|
|
06 /10/ 2022 |
Broken links replaced and update to COVID-19 testing requirements in line with Directors Letter (2022)32 |
|
|
18/11/2022 |
Updated to reflect that the advice contained within the Scottish Government’s DL(2022)10 remains extant. |
|
|
|
20/03/2023 |
Replaced with new Appendix 21 - COVID-19 Pandemic IPC for health and social care settings |
Appendix 22 - Community Infection Prevention and Control COVID-19 Pandemic Archive
Version |
Date |
Summary of changes |
|
29/06/2022 |
First publication – Marks transition from Winter Respiratory Infection IPC Addendum to a Community COVID-19 Pandemic Appendix. |
|
|
28/07/2022 |
GP surgeries included in this this appendix and removed from Appendix 21 – COVID-19 acute settings |
|
|
16/09/2022 |
Content revised to reflect SG Extended Use of Facemasks Policy for ASC settings. |
|
|
04/10/2022 |
Link added to revised content from SG clarifying use of facemasks in social care settings by healthcare staff |
|
|
18/11/2022 |
Updated to reflect that the advice contained within the Scottish Government’s DL(2022)10 remains extant. |
|
|
|
20/03/2023 |
Replaced with new Appendix 21 COVID-19 Pandemic IPC for health and social care settings. |
Appendix 21 - COVID-19 Pandemic IPC Controls for Health and Social Care Settings Archive
Version |
Date |
Summary of changes |
|
20/03/2023 |
New appendix which combines content from COVID-19 Appendix 21 for acute settings and Appendix 22 for community settings into a single pandemic appendix for health and social care settings. |
|
|
16/05/2023 |
Amendments to content made following Withdrawal of the Coronavirus (COVID-19): Extended Use of Face Masks and Face Coverings Guidance Across Health and Social Care Scottish Government DL (2023) 11. Renumbered as Appendix 19 on 2 June 2023 due to the archiving of outdated COVID-19 appendices. Appendix 19 was removed on 28 August 2023 due to changes in the SG COVID-19 testing procedures and the NIPCM was updated to reflect this. |
Hospital testing tables archive
Version |
Date |
Summary of changes |
|
23/12/2020 |
First version produced by Scottish Government |
|
|
22/02/2021 |
ARHAI Scotland taking responsibility for producing table. Inclusion of frontpage and review of content undertaken. |
|
|
18/05/2021 |
Update made to testing requirement 3 regarding emergency admissions and PCR/POCT testing. Update made to requirement 7 Transfer of a non COVID-19 patient to another hospital/NHS board about transferring patient while waiting for test results. Update to section Testing Contacts of confirmed COVID-19 cases. |
|
|
06/09/2021 |
Updates to sections on staff testing. Reference to household member testing positive removed. |
|
|
01/12/2021 |
Updated to be in line with the respiratory addendum. |
|
|
02/12/2021 |
Update to frontpage reference to discharge to care homes to include for those still in the 14 day isolation period. |
|
|
21/12/2021 |
Update to frontpage to include definition of admissions. Update to testing prior to an AGP to include further information on negative tests before an AGP. |
|
|
23/02/2022 |
Addition of relevant policy letters or guidance relevant to each testing recommendation. |
|
|
01/04/2022 |
Updates to reflect changes to healthcare COVID-19 pandemic controls outlined in DL (2022) 07 as follows
|
|
|
07/04/2022 |
Clarity relating to COVID-19 testing terminology |
|
|
25/04/2022 |
Additional clarifications relating to COVID-19 testing terminology |
|
|
10/05/2022 |
Changes to reflect transition of Winter Respiratory IPC addendum to COVID-19 appendix |
|
|
22/08/2022 |
Changes made to testing requirements in line with DL 2022(29) issued on 22nd August 2022 |
|
|
16/9/2022 |
Testing requirements updated with new paragraphs on symptomatic and asymptomatic testing. Addition of Appendix 1- List of PCR-based and non-PCR based tests in use in Scotland |
|
|
06/10/2022 |
Update to duration of precautions to reference NIPCM and A-Z |
|
|
|
|
The hospital testing tables were removed on 28 August 2023 due to changes in the SG COVID-19 testing procedures and the NIPCM was updated to reflect this. |
COVID-19 Literature Review Archive
All other literature reviews, rapid reviews and SBARs are held on the PHS COVID-19 Guidance Archive.
Winter preparedness campaign 2025
Every winter health and care providers experience additional demand for services due to an increase in seasonal viruses. These include:
These infections can spread quickly in areas such as hospital wards or care homes causing service users (for example patients and residents) and staff to become unwell.
Below you will find key infection prevention and control actions and supporting tools you can use in your winter planning preparations and during outbreaks and incidents. These should be used alongside the content of the National Infection Prevention and Control Manual (NIPCM) and Care Home Infection Prevention and Control Manual (CHIPCM).
It includes:
- winter planning preparation and communications
- incident and outbreak management
- seasonal review and improvement
It is essential that infection prevention and control is included within local winter plans.
Health and care organisations should assess local preparedness for the winter period and identify local actions needed to help relieve pressure points across the organisation to:
- prepare, plan and engage with staff before winter season starts
- implement lessons learned from the previous winter season
- improve data quality and reporting to support local and national assurance and understand when escalation is appropriate
- have an approach that acknowledges and responds to pressures across the organisation
Preparation and communications
To make sure that your health and care area is ready for winter you should have the correct products, procedures and communications in place to stop infections spreading.
Ensure ordering of supplies takes into account potential increase in product demand over winter period as well as seasonal holiday period and closures.
At all times Standard Infection Control Precautions (SICPs) should be followed. SICPs are the basic infection prevention and control measures necessary to reduce the risk of transmission of infectious agents from both recognised and unrecognised sources of infection.

SICPs may be not be enough to prevent cross-transmission of specific infections so you might also need to use additional precautions known as Transmission Based Precautions (TBPs) when caring for patients with a known or suspected infection or colonisation.
Further information on SICPs and TBPs can be found in the NIPCM and CHIPCM.
Make sure that you have the contact details for your local infection prevention and control (IPC) team and NHS board health protection (HPT) team so you can easily get advice and support on infection control and outbreak management during the winter season.
Communications
- Ensure key messages around respiratory and cough hygiene, and norovirus are effectively communicated to staff, service users and the public.
- Ensure that all national and local winter planning communications are available and circulated to staff.
- Provide appropriate communications on IPC and winter planning in in the care area. For example posters and signage in wards and staff kitchen areas promoting hand hygiene and respiratory and cough hygiene.
- Hand hygiene posters - Appendix 1 - How to hand wash and Appendix 2 - How to hand rub.
- Respiratory infections - key steps to stop the spread posters
- Norovirus - key steps to stop the spread posters
- Use infection alert posters to provide information on precautions to be taken when entering an area or visiting a service user.
- Have a supply of HAI and disease specific information leaflets to give to service users and visitors.
- Work with local communications team or staff to issue winter planning communications based on national messaging. This can include social media, websites, staff newsletters, infographics, posters, email signatures, videos and animations.
- Winter Preparedness Graphics used in this page
- Promote seasonal vaccinations for flu and COVID-19 to eligible groups using a range of communications materials, messaging and assets and NHS Inform.
- Winter programme 2025 - seasonal flu and COVID-19 vaccination (Scottish Government)
- Flu vaccination resources (Public Health Scotland)
- COVID-19 vaccination resources (Public Health Scotland)
- Health and social care workers - vaccination posters (Public Health Scotland)
- Winter vaccines campaign assets (Public Health Scotland)

- Remind staff:
- not to come into work if unwell and to use NHS Inform for further information on norovirus or respiratory infections
- not to attend work until 48 hours after their last symptom of diarrhoea and/or sickness
- about their eligibility for seasonal vaccinations
- to access training to ensure their IPC knowledge is up to date for example from NHS Education Scotland

- Remind visitors that they shouldn't visit if they:
- are feeling unwell
- have symptoms of a respiratory infection
- are not yet 48 hours symptom free of sickness and/or diarrhoea
- Direct the public to NHS Inform for further information on norovirus or respiratory infections.

Assessment of infection risk
- Infections can spread easily in busy areas so try to ensure that shared areas, for example waiting areas, are not overcrowded.
- Assess service users for infection risk on arrival to the care area (if possible, before transferring from another care area) and review throughout their stay.
- Follow your local risk assessment and admission screening policy.
- Use the Respiratory Symptom Screening Questions: Aide for Health and Social Care Settings.
- Apply hierarchy of controls to ensure staff and patient safety.
- Service users who present a particular cross-infection risk, for example with symptoms such as diarrhoea, sickness, fever or respiratory symptoms, should be isolated on arrival with appropriate clinical samples and screening undertaken as per national protocols to establish the causative pathogen.
- Use transmission based precautions as described in the NIPCM or CHIPCM.
- Use Appendix 11 for patient placement, PPE and RPE for specific infections.
- Use the A-Z of pathogens for more detailed information on specific pathogens and their notifiable status.
- In care homes use the Respiratory and Gastrointestinal resources and checklists.
- Ensure staff have access to appropriate training on patient placement and assessment for infection risk.
Hand hygiene
- Make sure there is an adequate supply of hand rub, soap and paper towels available to ensure hand hygiene can be correctly undertaken by service users, staff and visitors.

- Hand hygiene using soap and water should be used where a service user has symptoms of a gastro-intestinal infection (vomiting and/or diarrhoea).

- Remind visitors to use appropriate hand hygiene.
- Put up hand hygiene technique posters in areas where they are required for example hand rub dispensers at entrance to care area and above hand wash basins.
- Remind staff to undertake hand hygiene at the 5 moments or 4 moments in care homes.
- Make sure staff have access to appropriate training on hand hygiene
Respiratory and cough hygiene
- Staff should promote respiratory and cough hygiene helping those who need assistance with this, for example elderly and children.
- Provide service users with disposable tissues, plastic bags for used tissues and access to hand hygiene facilities as necessary.
- Encourage service users with symptoms of respiratory illness to wear a surgical (TYPE II R FRSM) face mask where it is clinically safe and tolerated by the wearer.
- Note: FRSM is not required when service user in their room on their own.

- Dispose of used tissues and face masks promptly into a waste bin.
- Wash hands with non-antimicrobial liquid soap and warm water after coughing, sneezing, using tissues, or after contact with respiratory secretions or objects contaminated by these secretions.
- Make sure staff have access to appropriate training on respiratory and cough hygiene.
Personal Protective Equipment (PPE)
- PPE is required to minimise the risk of cross-transmission of infection to yourself and others when providing care.
- PPE should be single-use items unless specified by the manufacturer whose decontamination procedures should be followed.
- Follow appropriate guidance for when and what PPE to wear when an infection is known or suspected.
- Make sure there is an adequate supply of all PPE that it is close to the point of use.
- Make sure staff have been fit tested before using RPE and know which make and model they have been fit tested for.
- Make sure staff perform a fit check every time they don a face filter piece 3 (FFP3) mask. Staff can access guidance on FFP3 masks in the NIPCM.
- PPE should be changed immediately after each service user and/or following completion of a procedure or task.
PPE should be disposed of after use into the correct waste stream which is healthcare waste or domestic waste.
Perform hand hygiene after removing PPE.
Advise visitors to wear PPE if they are performing direct care.
Make sure staff have access to appropriate training on PPE.
Care equipment and environment
- Make sure staff are aware of environmental and equipment cleaning schedules and know their specific responsibilities.
- Routine environmental cleaning should follow the guidance in the NHSScotland National Cleaning Specification and Cleaning Specification for Care Homes.
- Environmental and equipment decontamination may need to be increased when there is a known or suspected infection in the care area.
- There may be a need to update local cleaning schedules where increased frequency of environmental cleaning is required.
- If a patient or service user is suspected or known to have an infection, or where they are colonised with a transmissible pathogen, a Terminal clean of their room should be undertaken following transfer or discharge, or once the patient or service user is no longer considered infectious.
- Make sure staff have access to appropriate training on management of equipment and environment.
Incident and outbreak management
Norovirus and respiratory viruses can spread very quickly in hospitals and care homes and cause outbreaks. Outbreaks of infections can lead to:
- service users becoming unwell
- patients being in hospital longer than they should be
- ward, bay and care home closures
- staff absences
- increased bed pressures in the care areas
A Healthcare Associated Infection Outbreak can happen when there are:
- two or more linked cases with the same infectious agent associated with the same healthcare setting over a specified time period
- a higher-than-expected number of cases of HAI in a given healthcare area over a specified time period
An early and effective response to an actual or potential healthcare incident, outbreak or data exceedance is crucial. Contact your local infection prevention and control team (IPCT) if you think there may be an outbreak emerging in your area.
Care homes
Please note that depending on local processes, care homes should contact their local IPCT or HPT for support and advice regarding outbreaks in their care areas.
NHS boards
NHS Boards should follow Chapter 3 of the NIPCM Healthcare Infection Incidents, Outbreaks and Data Exceedance for incidents and outbreaks including for norovirus and respiratory infections.
Chapter 3 includes detailed definitions on outbreaks and incidents.
Local surveillance and reporting systems should be used for recognition and detection of potential healthcare infection incidents and outbreaks. Systems should make use of ‘triggers’ to allow prompt detection of any variance from normal limits.
Closely monitor rates of respiratory viruses including COVID-19 and provide infection prevention and control advice and guidance as required.
- Appendix 13 - NHSScotland Minimum Alert organism/Condition list should be used to establish and maintain local surveillance and reporting systems including development of triggers for clinical areas.
- Compliance monitoring of SICPs and TBPs
The Healthcare Infection Incident Assessment Tool (HIIAT) should be used by the IPCT or HPT to assess every healthcare infection incident and report all HIIAT assessed Green, Amber and Red reports to ARHAI Scotland through the electronic outbreak reporting tool (ORT).
Incidents assessed as Red, Amber or Green, where ARHAI support is requested, will be reviewed for onward communication to Scottish Government Healthcare Associated Infection Policy Unit.
- Respiratory incidents and outbreaks associated with key respiratory pathogens (COVID-19, influenza and respiratory syncytial virus (RSV)), should be completed within the Respiratory Short Form. However, where IPC measures do not align with the outbreak checklist and NIPCM, or where ARHAI support is requested a full ORT form must be completed.
- COVID-19 reporting should now align with reporting for other key respiratory pathogens (Influenza/RSV).
The Outbreak Checklist is designed to support staff with the prevention and control of suspected or confirmed incidents and outbreaks in hospital settings.
An incident/outbreak data collection tool template is available to help identify the total number of confirmed/probable/possible exposed cases.
Once the incident is declared over, and in addition to reporting via the electronic outbreak reporting tool (ORT), the IMT/NHS board should decide on the most appropriate format for a report to communicate any lessons learned from completing the Hot Debrief Tool.
- Note: The completed Hot Debrief Tool can be submitted to ARHAI Scotland. This is not mandatory, but for the purposes of sharing lessons learned across Scotland.
Review and improvement
After the winter season has ended it is important for health and care organisations to review how well the season went and include aspects of preparation, communications, outbreak and incident management. This can include measuring against:
- local and national audits
- local and national targets
- local and national policies
- feedback from staff, service users and visitors
It is important for organisations to celebrate successes and share achievements both locally and nationally.
Areas that can be considered for improvement include:
- staff education and training
- interpretation and application of necessary risk assessments
- control measures
- application of guidance documents
- application of NIPCM, CHIPCM and appendices
- interdependencies with other departments or organisations
- communications with staff, visitors and service users
The organisations multi-disciplinary team should actively consider learning opportunities presented by HAI data exceedance, incidents and outbreaks and disseminate, escalate, and share all relevant lessons learned via established local and national governance and reporting routes.
The Hot Debrief Tool can assist in sharing of lessons learned.
The results of these lessons learned should be used as recommendations for next years winter season and used as an improvement measure.
References
- Reference 1
The use of the word 'Persons' can be used instead of ‘Patient’ when using this document in non-healthcare settings.
Glossary
- Abrasion
A graze. A minor wound in which the surface of the skin or a mucous membrane has been worn away by rubbing or scraping.
- Acute care setting/Acute hospital
This is a unique, demanding and fast-paced environment designed to accommodate a wide variety of urgent, or emergent patient care needs.
- Adverse event
An event that could have caused or did result in harm to people or groups of people.
- Aerosol Generating Procedures (AGPs)
An AGP is a medical procedure that can result in the release of airborne particles from the respiratory tract when treating someone who is suspected or known to be suffering from an infectious agent transmitted wholly or partly by the airborne or droplet route.
- Aerosols
- Airborne (aerosol) transmission
The spread of infection from one person to another by airborne particles (aerosols) containing infectious agents.
- Airborne particles (aerosols)
Very small particles (of respirable size) that may contain infectious agents. They can remain in the air for extended periods of time and can be carried over long distances by air currents. Aerosols can be released during aerosol generating procedures (AGPs).
- Airborne precautions
A group of transmission-based precautions to prevent the spread of airborne pathogens.
- Alcohol based hand rub (ABHR)
A gel, foam or liquid containing one or more types of alcohol that is rubbed into the hands to inactivate microorganisms and/or temporarily suppress their growth.
- Alert organism
An organism that is identified as being potentially significant for infection prevention and control practices. Examples of alert organisms include Meticillin Resistant Staphylococcus aureus (MRSA), Clostridioides difficile (C.diff) and Group A Streptococcus.
- Alveolar
Refers to the alveoli which are the small air sacs in the lungs. Alveoli are located at the ends of the air passageways in the lungs, and are the site at which gas exchange takes place.
- Anteroom
An area with a door from or to the outside corridor and a second door giving access to the patient area (where both doors will never be open at the same time).
- Antimicrobial
An agent that kills microorganisms, or prevents them from growing.
Antimicrobials are grouped according to the microorganisms they act against, such as, antibiotics, antivirals, antifungals and antiparasitics.
- Antimicrobial hand wipes
Hand wipes that are moistened with an antimicrobial solution or agent at a concentration sufficient to inactivate microorganisms and/or temporarily suppress their growth.
- Antimicrobial resistance
The ability of a microorganism to resist the action of an antimicrobial drug or agent which could previously treat the infection caused by that microorganism.
- Antisepsis
The process of preventing infection by inhibiting the growth and multiplication of infectious agents. This is usually achieved by application of a germicidal preparation known as an antiseptic.
- Aseptic Technique
A healthcare procedure designed to minimise the risks of exposing the person being cared for to pathogenic micro-organisms during simple (for example dressing wounds) and complex care procedures (for example surgical procedures).
- Asymptomatic
Not showing any symptoms of disease but where an infection may be present.
- Augmented Care
In the context of infection prevention and control, most care designated as augmented will be that where medical or nursing procedures render the patients susceptible to invasive disease from environmental and opportunistic pathogens. However, there is no fixed definition of ‘augmented care’.
- Autoclave
Machine used for sterilising re-usable equipment using steam sterilisation. Re-usable equipment is exposed to steam at a required temperature, pressure, and time.
- Bay
A partly enclosed area within a ward containing one bed (single bay) or multiple beds (multi-bed bay).
- Blood Borne Viruses (BBVs)
Viruses carried or transmitted by blood and body fluids, for example Hepatitis B, Hepatitis C and HIV.
- Body Fluids
Fluid produced by the body such as urine, faeces, vomit or diarrhoea.
- British Standards (BS), European Standards (EN) and International Standards (ISO)
National standards specify the requirements for application in the particular country.
- BS denotes Britain's National Standards which are controlled by the British Standards Institute (BSI)
- EN denotes a Standard which is adopted by the European community and is controlled by the European Committee for Standardisation (CEN). Once a European Standard has been agreed it supersedes any existing national standard and becomes the new national standard. In Britain these Standards are then prefixed with BS EN
- ISO denotes a worldwide standard issued by the International Organisation for Standardisation. Once an International Standard has been adopted as a European Standard it supersedes the existing European standard. In Britain these Standards are then prefixed with BS EN ISO
- Care setting
Includes but is not limited to general practice, dental and pharmacy (primary care), acute care hospitals, emergency medical services, urgent-care centres and outpatient clinics (secondary care), specialist treatment centres (tertiary care), long-term care facilities such as nursing homes and skilled nursing facilities (community care), and care provided at home by professional healthcare providers (home care).
- Care staff
Any person who cares for patients, including healthcare support workers and nurses.
- Central Venous Catheter (CVC)
An intravenous catheter that is inserted directly into a large vein in the neck, chest or groin to give intravenous drugs, fluids and blood and to allow for quick medical tests.
- Chlorine
A chemical that is used for disinfecting, fumigating and bleaching.
- Cleaning
The removal of any dirt, body fluids (such as blood, vomit) by use of an appropriate cleaning agent such as detergent.
- Clinical wash hand basin
A sink designated for hand washing in clinical areas.
- Cohort area
A bay or ward in which a group of patients (cohort) with the same infection are placed. Cohorts are created based on clinical diagnosis, microbiological confirmation when available, epidemiology, and mode of transmission of the infectious agent.
- Colonisation
The presence of microorganisms on a body surface (such as the skin, mouth, intestines or airway) that does not cause disease or signs of infection in the person.
- Conjunctivae
Mucous membranes that cover the front of the eyes and the inside of the eyelids.
- Contact dermatitis
The inflammation of the skin (epidermis and adjacent dermis) resulting from direct contact of a substance that could either be an irritant (irritant contact dermatitis) or allergen (allergic contact dermatitis) with the surface of the skin.
- Contact precautions
Series of procedures/interventions used in addition to routine practices to prevent transmission of infectious agents that spread by direct or indirect contact.
- Contact transmission
The spread of infectious agents from one person to another by contact. When spread occurs through skin-to-skin contact, this is called direct contact transmission. When spread occurs via a contaminated object, this is called indirect contact transmission.
- Contaminated
The presence of an infectious agent on a body surface; also on or in clothes, bedding, surgical instruments or dressings, or other inanimate articles or substances including water and food.
- Cough etiquette/respiratory hygiene
Source control measures intended to contain respiratory secretions in order to limit transmission of respiratory pathogens.
- Cross-infection/Cross-transmission
Spread of infection from one person, object or place to another.
- Decontamination
The process of removing, or killing pathogens on an item or surface to make it safe for handling, re-use or disposal, by cleaning, disinfection and/or sterilisation.
- Detergent
A chemical cleansing agent that can dissolve oils and remove dirt.
- Diarrhoea
Passing looser more frequent stools than is normal for the individual.
- Direct contact transmission
Spread of infectious agents from one person to another by direct skin-to-skin contact.
- Disinfectant
A chemical used to reduce the number of infectious agents from an object or surface to a level that means they are not harmful to health.
- Disinfection
The treatment of surfaces or equipment using physical or chemical means, for example using a chemical disinfectant, to reduce the number of infectious agents from an object or surface to a level at which they are not harmful to health.
- Doffing
To remove (an item of clothing or an item of PPE).
- Domestic waste
Waste produced in the care setting that is similar to waste produced in the home.
- Donning
To put on (an item of clothing or an item of PPE).
- Droplet
A small drop of moisture, larger than airborne particle, that may contain infectious agents. Droplets can be released when a person talks, coughs or sneezes, and during some medical or patient care procedures such as open suctioning and cough induction by chest physiotherapy. It is thought that droplets can travel around 1 metre (3 feet).
- Droplet Nuclei
Droplet nuclei are aerosols formed from the rapid evaporation/desiccation of larger droplet particles when expelled/exhaled from the respiratory tract.
- Droplet transmission
The spread of infection from one person to another by droplets containing infectious agents.
- Emollient
A product used to moisturise the skin. It works by covering the skin with a protective film which traps in moisture.
- Enhanced single room (with en-suite facilities and ventilated lobby)
-
- Enhanced single room (with en-suite facilities)
-
- En-suite facilities
En-suite facilities should contain a shower, WC and a general wash-hand basin.
- En-suite single-bed room
A room with space for one patient with en-suite facilities.
- Exceptional infection episode
- Excretions
Waste products produced by the body such as urine and faeces (bowel movements).
- Exposure
The condition of being exposed to something that may have a harmful effect such as an infectious agent.
- Exposure Prone Procedures (EPPs)
Certain medical and patient care procedures where there is a risk that injury to the healthcare worker may result in exposure of the patient’s open tissues to the healthcare worker’s blood, for example the healthcare worker’s gloved hands are in contact with sharp instruments, needle tips or sharp tissues inside a patient’s body.
- Face covering
A term that applies collectively to items used to cover the nose and mouth. Also referred to as a face mask.
These should not be confused with items of PPE.
- Fallow time
The period of time required for droplets and/or aerosols to settle and be removed from the air following a procedure. It is also known as settle time.
- FFP3
Respiratory protection that is worn over the nose and mouth designed to protect the wearer from inhaling hazardous substances, including airborne particles (aerosols). FFP stands for filtering facepiece. There are three categories of FFP respirator: FFP1, FFP2 and FFP3. An FFP3 respirator or hood provides the highest level of protection, and is the only category of respirator legislated for use in UK healthcare settings.
- Fit check/seal check
A fit check, otherwise known as a user seal check, is a simple and quick method of checking that a respirator has been donned correctly to form a tight seal around the face. Fit checks are not substitutes for, and should not be confused with, fit testing.
- Fit Testing
A method to evaluate how well a tight-fitting respirator fits the wearer and seals adequately to their face. This process verifies the correct model, style, and size of respirator suitable for an individual.
- Fluid resistant surgical mask (FRSM)
- Fluid-resistant
A term applied to fabrics that resist liquid penetration, often used interchangeably with 'fluid-repellent' when describing the properties of protective clothing or equipment.
- Fomites
An inanimate substance or object that can transfer a pathogen to a host.
- Frequently touched surfaces
These are surfaces that are frequently touched by different people throughout the day and are therefore more likely to be contaminated with bacteria or viruses for example doorknobs, tables, phones, which can then easily transfer to the user.
- Germicide
An agent capable of destroying microorganisms, particularly organisms that are pathogenic.
- GP
General practitioner (your family doctor).
- Group 4 Infections
Definition taken from the HSE Approved list of biological agents www.hse.gov.uk/pubns/misc208.pdf
Group 4 infections cause severe human disease and are a serious hazard to employees; they are likely to spread to the community and there is usually no effective prophylaxis or treatment available.
- Hand Hygiene
The process of decontaminating your hands using either hand rub or liquid soap and water.
- Hand rub
A gel, foam or liquid containing one or more types of active ingredient that is rubbed into the hands to inactivate microorganisms and/or temporarily suppress their growth. A hand rub should meet the required British or European Standards as defined in the hand products literature review.
- Health Protection Team (HPT)
A team of healthcare professionals whose role it is to protect the health of the local population and limit the risk of them becoming exposed to infection and environmental dangers. Every NHS board has a HPT.
- Healthcare Associated Infection (HAI)
Infections that are not present or incubating at the time of admission, and occur as a result of medical care, or treatment, in any healthcare setting.
See nosocomial.
- Healthcare associated infection outbreak
See Incident/Outbreak.
- Healthcare infection data exceedance
- Healthcare infection exposure incident
See Incident/Outbreak.
- Healthcare infection near miss incident
See Incident/Outbreak.
- Healthcare Waste
All waste produced as a result of healthcare activities.
- Hierarchy of controls
This is a systematic process which provides a consistent approach to minimising or eliminating exposures to hazards in the workplace.
- High Consequence Infectious Disease (HCID)
A High Consequence Infectious Disease (HCID) is defined according to the following criteria:
- causes acute infectious disease
- typically has a high case-fatality rate
- may not have effective prophylaxis or treatment
- difficult to recognise and detect quickly
- ability to spread in the community and within healthcare settings
- requires an enhanced individual, population, and system response for safe, efficient, and effective management
Previously referred to as an Infectious Diseases of High Consequence (IDHC).
- Hospital infection incident assessment tool (HIIAT)
Used by the IPCT or HPT to assess every healthcare infection incident, meaning all outbreaks and incidents including decontamination incidents or near misses in any healthcare setting, (that is the NHS, independent contractors providing NHS services and private providers of healthcare).
- Hypochlorite
A chlorine-based disinfectant such as bleach.
- Immunisation
To provide immunity to a disease by giving a vaccination.
- Immunocompromised patient/individual
Any person whose immune response is reduced or deficient, usually because they have a disease or are undergoing treatment. People who are immunocompromised are more vulnerable to infection.
- Impervious
Cannot be penetrated by liquid.
- Incident Management Team (IMT)
A multidisciplinary, multi-agency group with responsibility for investigating and managing an incident.
- Incident/outbreak
An incident/outbreak may be:
- An exceptional infection episode, defined a single case of rare infection that has severe outcomes for an individual AND has major implications for others (patients, staff and/or visitors), the organisation or wider public health for example, high consequence infectious disease (HCID) OR other rare infections such as XDR-TB, botulism, polio, rabies, or diphtheria.
- A healthcare infection exposure incident, defined as exposure of patients, staff, public to a possible infectious agent as a result of a healthcare system failure or a near miss, for example ventilation, water or decontamination incidents.
- A healthcare associated infection outbreak, defined as two or more linked cases associated with the same infectious agent, within the same healthcare setting, over a specified time period, or a higher-than-expected number of cases in a given healthcare area over a specified time period.
- A healthcare infection data exceedance, defined as a greater than expected rate of infection compared with the usual background rate for the place and time where the incident has occurred.
- A healthcare infection near miss incident, which had the potential to expose patients to an infectious agent but did not, for example decontamination failure.
- Indirect contact transmission
The spread of infectious agents from one person to another via a contaminated object.
- Infection
Invasion of the body by a harmful organism or infectious agent such as a virus, parasite, bacterium or fungus.
- Infection Prevention and Control Team (IPCT)
A multidisciplinary team responsible for preventing, investigating and managing an infection incident or outbreak.
- Infectious agent
Any organism, such as a virus, parasite, bacterium or fungus, that is capable of causing an infection or infectious disease.
- Infectious period
The time when an infectious agent may be transmitted directly or indirectly from an infected person to another person. Also known as “period of infectiousness” and “communicability”.
- Inpatient
A patient is termed an inpatient when they occupy a staffed bed in a hospital and either remains overnight (whether intended or not), or is expected to remain overnight but is discharged earlier. An inpatient’s admission can be an emergency, an elective or as a transfer.
- Invasive device
A device which penetrates the body, either through a body cavity or through the surface of the body. Central Venous Catheters (central line), Peripheral Arterial Lines and Urinary Catheters are examples of invasive devices.
- Invasive procedure
A medical or healthcare procedure that penetrates or breaks the skin or enters a body cavity.
- Isolation
Physically separating patients to prevent the spread of infection.
- Isolation suite/room
An isolation suite/room consists of enhanced en-suite single bedrooms.
An en-suite single bedroom is defined as: consisting of a bed, locker or wardrobe, clinical wash-hand basin and en-suite shower, WC and wash-hand basin. (In new build, space for a social support zone for overnight stay and a clinical support zone is also provided).
- Enhanced single room (with en-suite facilities), also called isolation room, is the same as an en-suite single-bed room but with a ventilation system that prevents uncontrolled escape of infectious aerosols from the room to adjacent areas. It can also provide a degree of dilution of infectious aerosols in the room for the safety of staff and visitors. The room should have extract ventilation that exceeds its supply, such that gaps in its fabric leak inwards not outwards.
- Enhanced single room (with en-suite facilities and ventilated lobby), also called isolation suite, is the same as an enhanced single room (with en-suite facilities) but with a lobby having positive pressure ventilation.
- J
No terms
- K
No terms
- Long Term Care Facility (LTCF)
Long term care facilities provide a variety of services, both medical and personal care, to people who are unable to live independently.
- Mechanical Ventilation
Mechanical ventilation brings fresh air into a building from outside via a controllable method. Basic systems consist of a fan and either collection (extraction) or distribution (supply) ductwork.
- Microorganism (microbe)
Any living thing (organism) that is too small to be seen by the naked eye. Bacteria, viruses and some parasites are microorganisms.
- Mode of transmission
The way that microorganisms spread from one person to another. The main modes or routes of transmission are airborne (aerosol) transmission, droplet transmission and contact transmission.
- Mucocutaneous exposure
An incident in which the mucous membranes (for example mouth, nose, eyes) or non-intact skin have been contaminated with blood or other bodily fluids.
- Mucous membranes/mucosa
The surfaces lining the cavities of the body that are exposed to the environment such as the lining of the mouth and nose.
- Multi-bed room
A room that contains more than one bed.
The acceptable maximum number of beds in a multi-bed room is four. Multi-bed rooms require two clinical wash-hand basins and must have en-suite sanitary facilities. Ideally, an assisted shower room (with WC, shower and general wash-hand basin) and a separate semi-ambulant WC (with general wash-hand basin) both en-suite.
- Negative pressure isolation room
A room with a high ventilation rate that maintains permanent negative pressure through a controlled direction of airflow. Air flow is from the outside adjacent space (for example corridor) into the room, then exhausted to the outside. It may also be known as an airborne infection isolation room (AIIR).
The room should be used to accommodate a patient known or suspected to be infected with a microorganism spread by the airborne route whilst the patient is considered infectious.
- Non-intact skin
Skin that is broken for example by cuts, abrasions, dermatitis, chapped skin, eczema.
- Non-intact skin exposure
An incident in which non-intact skin is exposed to blood or body fluids.
- Non-sterile procedure
Care procedure that does not need to be undertaken in conditions that are free from bacteria or other microorganisms.
- Nosocomial
An infection occurring in a patient during the process of care in a hospital or other health care facility, which was not present or incubating at the time of admission.
- Novel
Scientifically, novel is used to refer to things of recent origin or introduction, such as a novel pathogen.
- Occupational exposure
An occupational exposure is a percutaneous or mucocutaneous exposure to blood or other body fluids.
- Organism
Any living thing that can grow and reproduce, such as a plant, animal, fungus or bacterium.
- Outbreak
- Outpatient
An outpatient is a patient who attends a consultant or other medical/healthcare clinic or has an arranged meeting with a consultant or a senior member of their team out with a clinic session. Outpatient attendances involve treatment or assessment that only take a short time to complete. Outpatient attendances are categorised as new or return (follow-up).
- Overcrowding
Within health and care settings, this is the state of being filled past capacity or comfort and therefore being burdened by excessive demands for services.
- Pandemic
A disease outbreak that occurs over a wide geographical area (such as multiple countries and/or continents) and typically affects a significant proportion of the population.
- Pathogen
Any disease-producing infectious agent.
- Patient cohorting
Placing a group of two or more patients (a cohort) with the same infection or strain in the same bay or ward. Cohorts are created based on clinical diagnosis, microbiological confirmation, epidemiology, and mode of transmission.
- PCR test
PCR test is a highly accurate test used to diagnose certain infectious diseases.
- Percutaneous injury
An injury caused by a needle or sharp, instrument, bone fragment, human scratch or bite cutting or puncturing the skin.
- Personal Protective Equipment (PPE)
Equipment a person wears to protect themselves from risks to their health or safety, including exposure to infections, for example disposable gloves and disposable aprons.
- Physical Distancing
Keeping a distance from other people, in order to stop transmission of a disease to another person or other people.
- Positive pressure isolation room
A specialised room with a high ventilation rate, that is maintained at positive pressure relative to the surrounding environment. This means the airflow is directed away from the room into the surrounding area, preventing the entry of contaminated air. Where possible, the air entering the room should be HEPA filtered. It is used to minimise exposure of severely immunocompromised patients to opportunistic airborne infectious agents.
- Positive pressure ventilated lobby (PPVL) Suite
A PPVL suite is a specialised single-patient room with an entry lobby, maintained at a higher air pressure than the room itself and the surrounding areas. The positive pressure in the lobby forces air outward, preventing air from the corridor from entering the room and stopping air from the room from escaping into the corridor. These suites are used for patients who are severely immunocompromised and need protection from airborne pathogens, while also requiring containment because they may be infectious to others.
- Pre-symptomatic
The time period when someone has the infection but has not yet developed symptoms but does go on to develop symptoms later in the disease.
- Primary Care setting
These provide the first point of contact in the healthcare system and includes general practice, dentistry, community pharmacies.
- Problem Assessment Group (PAG)
A group that is convened by the Infection Prevention and Control Team (IPCT) or Health Protection Team (HPT) to assess a healthcare incident/outbreak/data exceedance and determine if further action is required.
The assessment and outcome may be:
- HIIAT Green - continue to monitor
- HIIAT Amber or Red - IMT required
- Pseudo-outbreak
A pseudo-outbreak describes a situation in which there is an increased rate of microorganisms identified in clinical patient samples, without evidence of colonisation or infection within the patient.
Pseudo-outbreaks may be caused by several factors, including contaminated equipment and water supply systems.
- Q
No terms
- Recapping/Re-sheathing
To put a needle or other sharp object back into its plastic sheath or cap. Also known as ‘re-sheathing’.
- Receptacle
Container or bag that is used to hold or store healthcare waste for disposal.
- Respiratory droplets
A small droplet >5 μm in diameter, such as a particle of moisture released from the mouth during coughing, sneezing, or speaking.
- Respiratory Protective Equipment (RPE)
Respirators are devices that cover the nose and mouth and are designed to filter the air breathed in to protect the wearer from inhaling hazardous substances.
They provide respiratory protection from infectious agents transmissible by the airborne (aerosols) route. FPP3 respirators are recommended for use in UK health and care settings when exposure to aerosols is anticipated.
- Safer sharp
A medical sharps device which has been designed to incorporate a feature or mechanism that minimises and/or prevents the risk of accidental injury. Other terms include (but are not limited to) safety devices, safety-engineered devices and safer sharps devices.
- Safer sharps device
Any device designed to reduce the risk of injury from needles. This may include needle-free devices or mechanisms on a needle, such as an automated resheathing device, that cover the needle immediately after use.
- Sanitary fittings
All sinks and furniture in a bathroom, including a toilet, bath, shower.
- Screening
Performing a test or enquiry to identify individuals at risk of a specific disorder or infection to warrant further investigation or direct preventive action.
- Secondary care setting
Secondary care settings are those to which patients are referred for specialised treatment (for example routine surgeries, rehabilitation, therapists, mental healthcare) or urgent and emergency care (including 999 and 111 services, ambulance services, hospital emergency departments, out-of-hours GP services) that cannot be provided in primary care settings.
Secondary care is usually provided in hospitals but may also be community-based.
- Secretions
Any body fluid that is produced by a cell or gland such as saliva or mucous, for a particular function in the organism or for excretion.
- Segregated
Physically separated or isolated from other people.
- Sepsis
A life-threatening condition that arises when the body’s immune system responds to an infection, for example pneumonia (lung infection), injures its own tissues and organs. This can lead to multiple organ failure and death. Early recognition, treatment and management is key to successful patient outcomes.
- Sharp
A ‘sharp’ is a device or instrument used in healthcare settings with sharp points or edges, such as needles, lancets and scalpels which have the potential to cause injury through cutting or puncturing the skin.
- Sharps incident
-
A type of percutaneous injury caused by a sharp instrument or device which cuts or penetrates the skin.
- Sharps injury
See percutaneous injury.
- Significant occupational exposure
A percutaneous, mucocutaneous or non-intact skin (abrasions, cuts, eczema) exposure to blood or other body fluids from a source that is known (or later found to be) positive for a bloodborne virus infection.
- Significant sharps incident
An incident which involves a used needle that has exposed, or may have exposed, the employee to blood or body fluids.
- Single-bed room
A room with space for one patient and usually contains as a minimum: a bed, locker or wardrobe, clinical wash-hand basin.
Single-bed rooms should also have en-suite sanitary facilities comprising of a shower, WC and a general wash-hand basin.
- Skin integrity
Skin being intact, and in an unimpaired condition.
- Source control
This term encompasses all physical measures used to prevent or control the transmission of an infectious agent, applied to individuals at risk of spreading it. Such measures include:
- isolating infected individuals
- patients’ and service users use of respirators or well-fitting masks (when indicated) to prevent the spread of respiratory secretions when they are breathing or coughing
- use of headwear and gowns by healthcare staff to prevent contamination of the surgical field
- Spore
A reproductive cell produced by fungi and some types of bacteria under certain environmental conditions. Spores can survive for long periods of time and are very resistant to heat, drying and chemicals.
- Staff cohorting
An IPC practice of dedicating a team of healthcare staff to care exclusively for a cohort of patients over a specific period.
- Standard infection control precautions (SICPs)
These are a set of basic infection prevention and control practices that must be adopted by all staff in health and care settings, irrespective of infectious status of patient.
- Sterile
Free from live bacteria or other microorganisms.
- Sterile procedure
Care procedure that is undertaken in conditions that are free from bacteria or other microorganisms.
- Sterilisation
The procedure of making some object free of all germs, live bacteria or other microorganisms (usually by heat or chemical means).
- Surgical face mask
A disposable fluid-resistant mask worn over the nose and mouth to protect the mucous membranes of the wearer’s nose and mouth from splashes and infectious droplets and to protect patients. When recommended for infection control purposes a 'surgical face mask' typically denotes a fluid-resistant (Type IIR) surgical mask.
- Surgical hand antisepsis
Surgical scrubbing or surgical rubbing. More thorough than routine hand hygiene. In addition to the removal of visible soiling and transient bacteria, it prevents the growth of resident microbial skin flora before performing an invasive procedure.
- Surgical rubbing
The process of surgical hand antisepsis using ABHR prior to performing a sterile or surgical procedure. An alternative to surgical scrubbing when hands are not visibly soiled.
- Surgical scrubbing
The process of removing debris and sterilising hands prior to performing a sterile or surgical procedure.
- Surgical site infection
This is an infection that occurs after surgery at the site of the surgical incision due to introduction and multiplication of pathogens at the surgical site.
- Swan-neck knot
A method of closing a bag by twisting the top of the bag (must not be more than 2/3 full), looping the neck back on itself, holding the twist firmly, and placing a seal over the neck of the bag (such as with a tag).
- Terminal decontamination
Cleaning or decontamination of the environment following transfer or discharge of a patient, or when they are no longer considered infectious, to ensure the environment is safe for the next patient or for the same patient on return.
- Transmission-based precautions (TBPs)
These are additional measures that are used in conjunction with SICPs when caring for patients with a known or suspected infection or colonisation.
- U
No terms
- Vaccination
Treatment with a vaccine to produce immunity against a disease.
- Vaccine
A suspension that is administered in order to stimulate the immune response of the body against an infectious agent.
- Vascular access devices
Any medical instrument used to access a patient’s veins or arteries such as a Central Venous Catheter or Peripheral Vascular Catheter.
- Ventilation
Ventilation is a means of removing and replacing the air in a space. In its simplest form this may be achieved by opening windows and doors.
- Viral load
The viral load or viral burden is a numerical expression of the amount of virus present in biological fluids or environmental specimens.
- Ward
An area forming a division of a care setting (or a suite of rooms) shared by patients who need a similar type of care.
- X
No terms
- Y
No terms
- Z
No terms